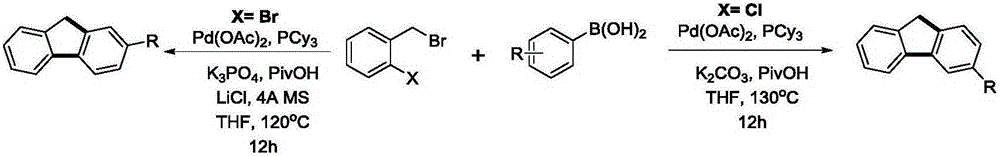Preparation method of all-position replacement fluorene compounds obtained through halogen adjustment and control
A kind of fluorene compound, halogen technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
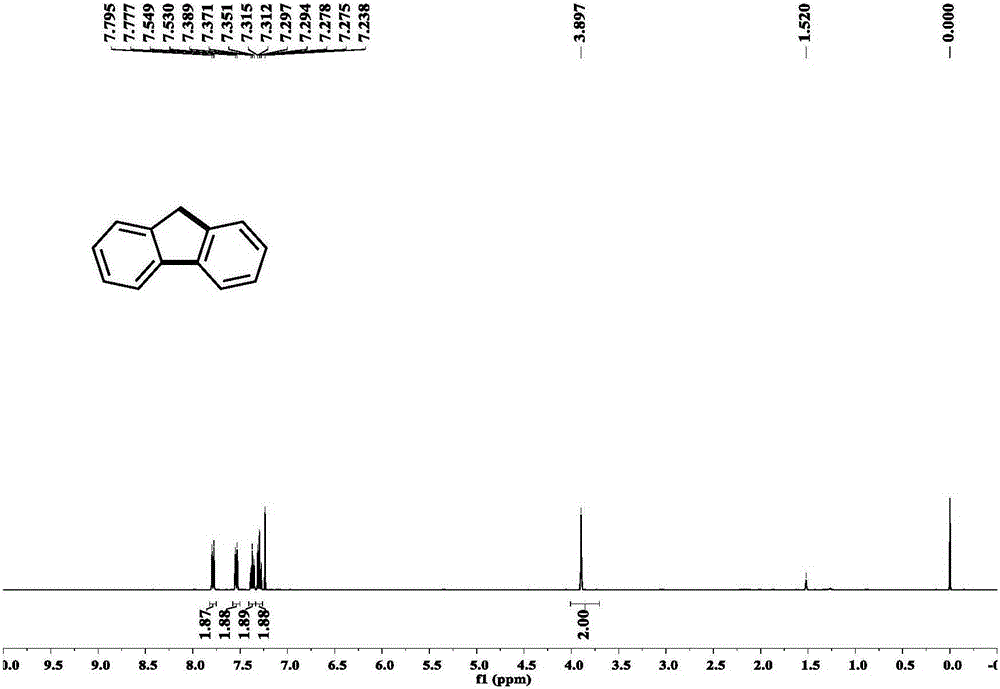
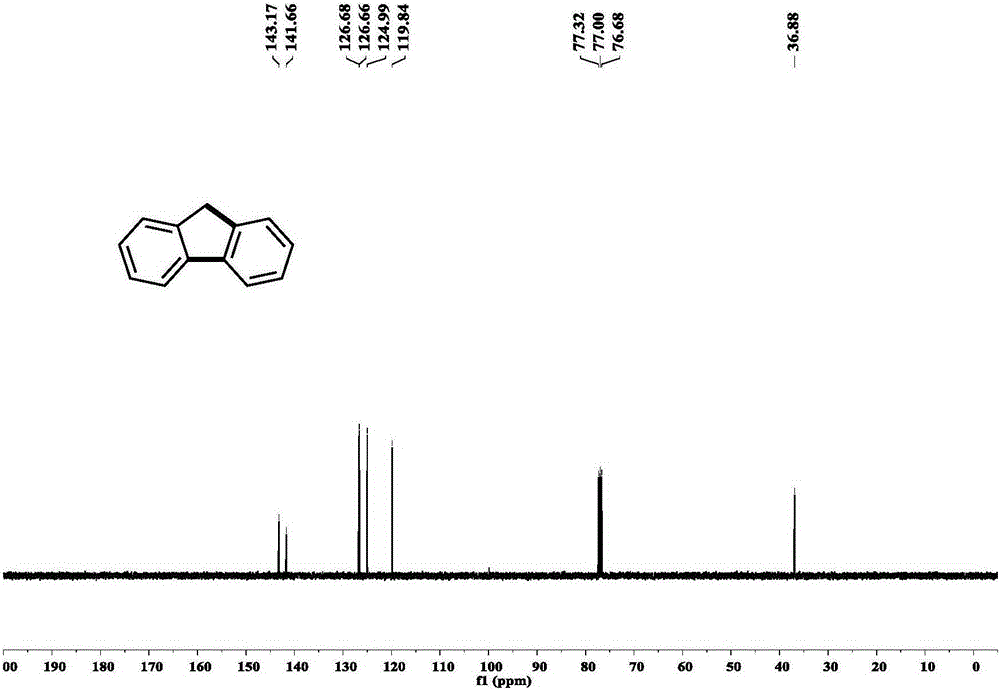
[0053] Example 1: Under nitrogen protection, add phenylboronic acid (0.45mmol, 54.9mg) and palladium acetate (0.009mmol, 0.28mg) in a Schlenk reaction tube, in the glove box, add potassium phosphate (1.8mmol, 382.0mg) , tricyclohexylphosphine (0.018mmol, 5.0mg), lithium chloride (0.45mmol, 19.1mg), Molecular sieves (200 mg). Add o-bromobenzyl bromide (0.3mmol, 75mg) and pivalic acid (0.24mmol, 24.5mg) to the system successively under a nitrogen atmosphere, then add 5ml of tetrahydrofuran, stir for 5 minutes, and place the reaction React in a pot at 120°C for 12 hours. After the reaction was over, 3 milliliters of saturated ammonium chloride solution was added to the system to quench the reaction, and 15 milliliters of ethyl acetate was added for extraction three times. The organic phases were combined, dried over anhydrous sodium sulfate, and separated by column chromatography to obtain the product with a yield of 85%. %. Such as figure 2 , 3 shown.
[0054] In embodim...
Embodiment 11
[0058] Example 11: Under nitrogen protection, add phenylboronic acid (0.33mmol, 40.2mg) in a Schlenk reaction tube, palladium acetate (0.009mmol, 0.28mg), in the glove box, add potassium carbonate (1.8mmol, 248.8mg) , Tricyclohexylphosphine (0.018 mmol, 5.0 mg). Add o-chlorobenzyl bromide (0.3mmol, 61.7mg) and pivalic acid (0.3mmol, 30.6mg) to the system successively under a nitrogen atmosphere, then add 2ml of tetrahydrofuran, stir for 5 minutes, and react Placed in a pot at 130°C for 12 hours. After the reaction was over, 3 milliliters of saturated ammonium chloride solution was added to the system to quench the reaction, and 15 milliliters of ethyl acetate was added for extraction three times. The organic phases were combined, dried over anhydrous sodium sulfate, and separated by column chromatography to obtain the product with a yield of 62 %. Such as Figure 22 , 23 shown.
Embodiment 12-18
[0059] Example 12-18: Under nitrogen protection, arylboronic acid (0.33mmol), palladium acetate (0.009mmol, 0.28mg) was added in a Schlenk reaction tube, and potassium carbonate (1.8mmol, 248.8mg) was added in the glove box , Tricyclohexylphosphine (0.018 mmol, 5.0 mg). Add o-chlorobenzyl bromide (0.3mmol, 61.7mg) and pivalic acid (0.3mmol, 30.6mg) to the system successively under a nitrogen atmosphere, then add 2ml of tetrahydrofuran, stir for 5 minutes, and react Placed in a pot at 130°C for 12 hours. After the reaction was completed, 3 ml of saturated ammonium chloride solution was added to the system to quench the reaction, and 15 ml of ethyl acetate was added three times for extraction. The organic phases were combined, dried over anhydrous sodium sulfate, and separated by column chromatography to obtain the product. Such as Figure 24 to figure Figure 37 shown.
[0060] Table 2: Reaction of o-chlorobenzyl bromide with arylboronic acids.
[0061]
[0062] The pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com