Synthesis method of related polypeptide
A synthetic method and type of technology, applied in the field of peptide-like synthesis, can solve the problems of limitations, harshness, and unsuitability for large-scale synthesis, and achieve the effect of facilitating polymerization and increasing the reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
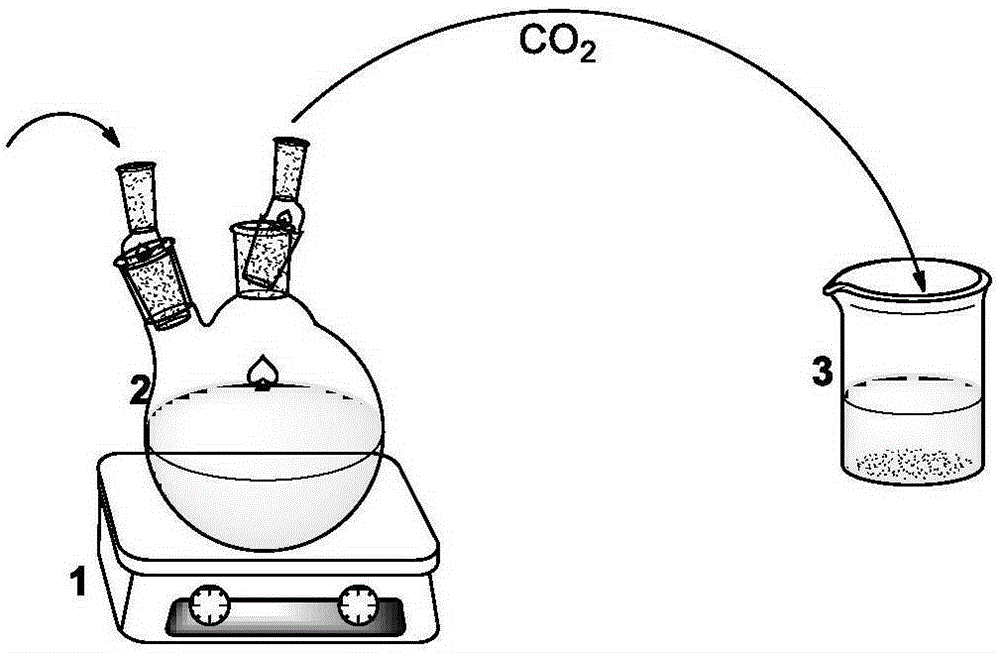
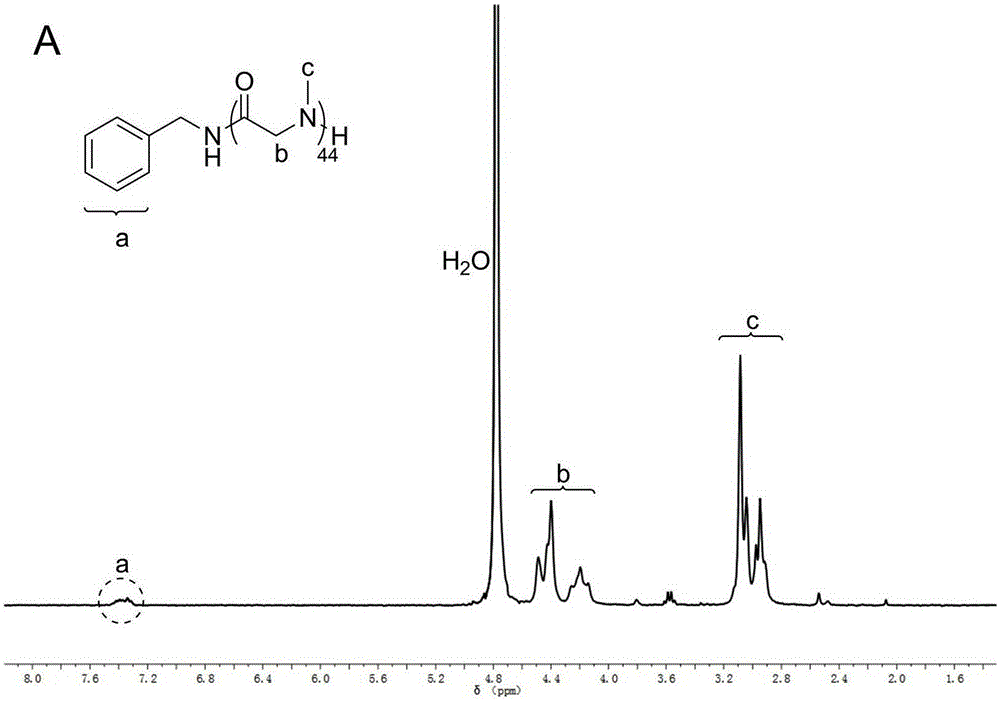
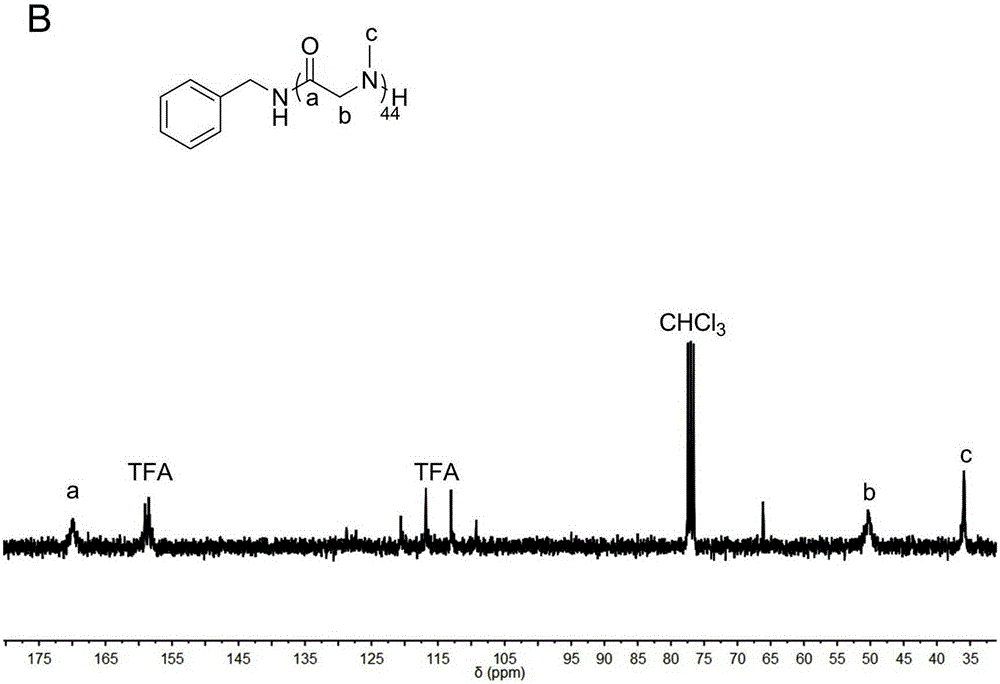
[0035] Such as figure 1 As indicated, under the condition of nitrogen flow, N-methyl substituted glycine-N-carboxy anhydride (0.2581g, 2.3mmol) was dissolved in 2.3mL of anhydrous acetonitrile, after complete dissolution, benzylamine (10uL, 0.092mmol) added to the solution ([monomer] 0 / [initiator 0 ]=25). The reaction solution was reacted at room temperature under nitrogen flow. After reacting for 4 hours, the reaction liquid was added into excess diethyl ether to precipitate a polymer, and then the polymer was vacuum-dried. polymer structure through 1 HNMR with 13 CNMR identification (see figure 2 and image 3 ), the molecular weight and degree of dispersion of the polymer are measured by GPC (gel permeation chromatography). GPC test conditions: flow rate 1mL / min, mobile phase DMF, temperature 50°C. From Figure 4 In the reaction kinetic curve, we found that the reaction rate was significantly increased under the action of nitrogen flow. (number average molecular...
Embodiment 2
[0037] Under the condition of nitrogen flow, N-methyl-substituted glycine-N-carboxy anhydride (3.097g, 27.6mmol) was dissolved in 27.6mL of anhydrous dichloromethane, after complete dissolution, propylamine (7.5uL, 0.092 mmol) was added to the solution ([monomer] 0 / [initiator 0 ]=300). The reaction solution was reacted at room temperature under nitrogen flow. After reacting for 4 hours, the reaction liquid was added into excess diethyl ether to precipitate a polymer, and then the polymer was vacuum-dried. polymer structure through 1 HNMR with 13 CNMR identification, molecular weight and dispersion of the polymer are measured by GPC (gel permeation chromatography). GPC test conditions: flow rate 1mL / min, mobile phase DMF, temperature 50°C. (number average molecular weight 20.7 kg / mol; molecular weight distribution 1.08; yield 92.1%).
Embodiment 3
[0039] Under nitrogen flow conditions, N-ethyl substituted glycine-N-carboxy anhydride (1.1860g, 9.2mmol) was dissolved in 9.2mL of anhydrous acetonitrile, after complete dissolution, benzylamine (10uL, 0.092mmol) added to the solution ([monomer] 0 / [initiator 0 ]=100). The reaction solution was reacted at room temperature under nitrogen flow. After reacting for 8 hours, the reaction liquid was added into excess diethyl ether to precipitate the polymer, and then the polymer was vacuum-dried. polymer structure through 1 HNMR with 13 CNMR identification, molecular weight and dispersion of the polymer are measured by GPC (gel permeation chromatography). GPC test conditions: flow rate 1mL / min, mobile phase DMF, temperature 50°C. (number average molecular weight 1.8 kg / mol; molecular weight distribution 1.22; yield 86.1%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com