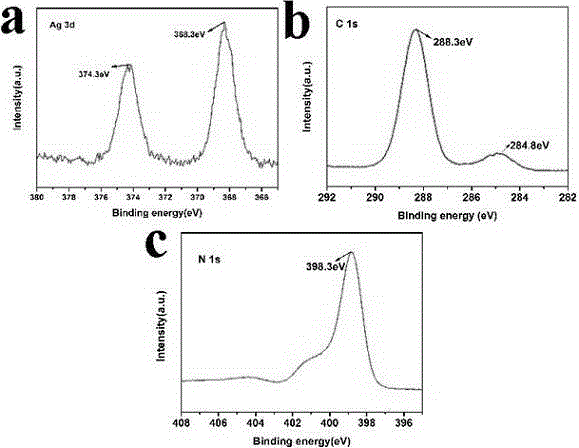
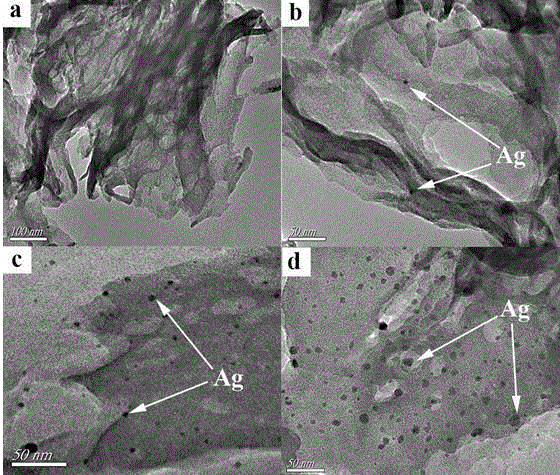
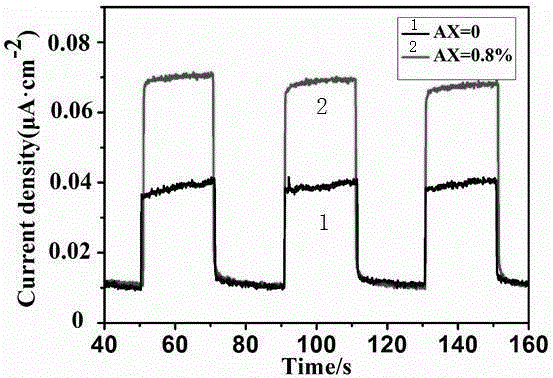
Method for preparing Ag/g-C3N4 catalyst
A g-c3n4, catalyst technology, applied in physical/chemical process catalysts, chemical instruments and methods, hydrogen production, etc., can solve problems such as influence and reduce photocatalytic efficiency, and achieve simple process, good repeatability, and yield. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A. Weigh 10 g of urea and dissolve it in 40 ml of distilled water and ultrasonicate for 20 minutes to obtain solution A.
[0029] B. Heat the obtained solution A in a water bath at 90°C with constant stirring until it evaporates to dryness quickly, and then put it into an oven at 60°C to dry to obtain sample B.
[0030] C. Put sample B in a round crucible, cover the crucible and place it horizontally in the muffle furnace, ensuring that the initial temperature of the muffle furnace is less than 80 degrees Celsius.
[0031] D. Raise the temperature of the muffle furnace to 550°C at a rate of 2.3°C per minute, and keep it at this temperature for 4 hours to obtain sample C.
[0032] E. Naturally cool to room temperature, wash sample C with deionized water and absolute ethanol three times respectively, and then dry in an oven at 60°C for 12 hours to obtain pure g-C 3 N 4 .
Embodiment 2
[0034] A. Weigh 10 g of urea and dissolve it in 40 ml of distilled water and ultrasonicate for 20 minutes to obtain solution A.
[0035] B. Add 0.03gAgNO 3 Added to solution A, and stirred in the air for 20min to obtain solution B.
[0036] C. Heat the obtained solution B in a water bath at 90°C with constant stirring until it evaporates to dryness quickly, and then dry it in an oven at 60°C to obtain sample C.
[0037] D. Put sample C in a circular crucible, cover the crucible and place it horizontally in the muffle furnace to ensure that the initial temperature of the muffle furnace is less than 80 degrees Celsius.
[0038] E. Raise the temperature of the muffle furnace to 550°C at a rate of 2.3°C per minute, and keep it at this temperature for 4 hours to obtain sample D.
[0039] F. Naturally cool to room temperature, wash sample D with deionized water and absolute ethanol 3 times respectively, and then dry in a 60°C oven for 12h to obtain Ag quantum dot-modified g-C 3 N...
Embodiment 3
[0041] A. Weigh 10 g of urea and dissolve it in 40 ml of distilled water and ultrasonicate for 20 minutes to obtain solution A.
[0042] B. Add 0.08gAgNO 3 Added to solution A, and stirred in the air for 20min to obtain solution B.
[0043]C. Heat the obtained solution B in a water bath at 90°C with constant stirring until it evaporates to dryness quickly, and then dry it in an oven at 60°C to obtain sample C.
[0044] D. Put sample C in a circular crucible, cover the crucible and place it horizontally in the muffle furnace to ensure that the initial temperature of the muffle furnace is less than 80 degrees Celsius.
[0045] E. Raise the temperature of the muffle furnace to 550°C at a rate of 2.3°C per minute, and keep it at this temperature for 4 hours to obtain sample D.
[0046] 、 Naturally cooled to room temperature, sample D was washed three times with deionized water and absolute ethanol, and then dried in an oven at 60°C for 12 h to obtain Ag quantum dot-modified g-C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com