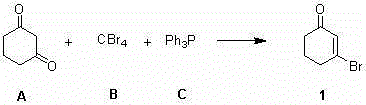Synthesis method of 3-bromocyclohex-2-enone
A synthesis method and technology of cyclohexanedione, which is applied in the field of preparation of pharmaceutical intermediates, can solve problems such as unfavorable industrial production, difficult control of reaction safety, harsh reaction conditions and production equipment requirements, and achieve easy control of safety and equipment requirements. The effect of low and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] A kind of synthetic method of 3-bromocyclohex-2-en-1-one, comprises the steps:
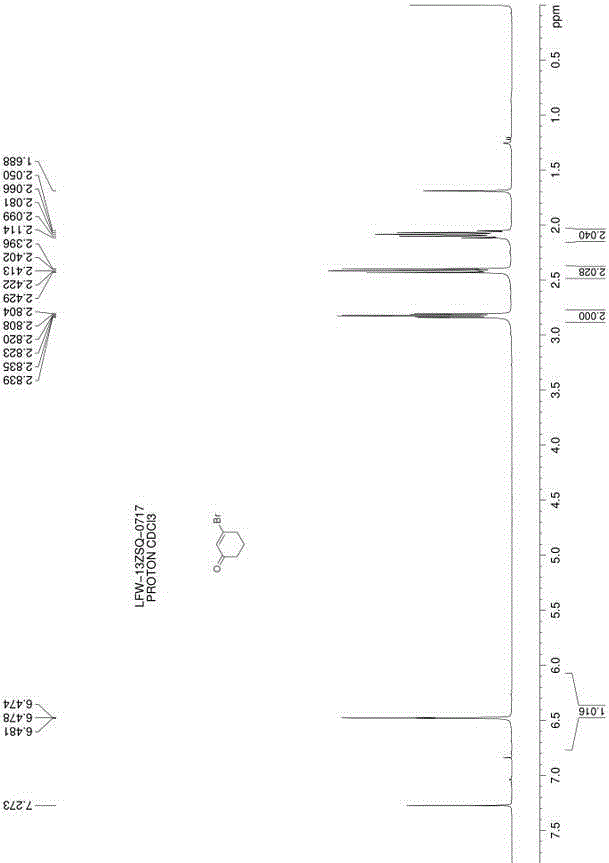
[0018] Dissolve 17.7g (1.2eq.) of carbon tetrabromide in 10mL of toluene; take a 100mL three-necked flask, add 30mL of toluene, and stir, mix 5.0g (1.0eq.) of 1,3-cyclohexanedione and 11.7g Add (1.0eq.) triphenylphosphine into the three-necked flask, replace the nitrogen protection, cool down to 0-5°C, slowly add the toluene solution of carbon tetrabromide into the three-necked flask; dropwise, at 0-5°C Stir the reaction for 12 hours; filter the reaction solution, wash the filter cake 3 times with n-hexane, combine the filtrate, concentrate the filtrate to about 15 mL, and use a silica gel chromatography column (the eluent is petroleum ether: ethyl acetate volume ratio = 8:1) 7.5 g of 3-bromocyclohex-2-en-1-one was isolated as a colorless liquid with a yield of 96%. h 1 NMR (500MHz, CDCl 3 ) δ (ppm) 6.47 (m, 1H), 2.83 (m, 2H), 2.42 (m, 2H), 2.09 (m, 2H).
Embodiment 2
[0020] A kind of synthetic method of 3-bromocyclohex-2-en-1-one, comprises the steps:
[0021] Dissolve 41.4g (2.0eq.) of carbon tetrabromide in 15mL of cyclohexane; take a 100mL three-necked flask, add 40mL of cyclohexane, and stir to dissolve 7.0g (1.0eq.) of 1,3-cyclohexane Add ketone and 33.0g (2.0eq.) triphenylphosphine into the three-necked flask, replace the argon protection, control the temperature at 25-30°C, and slowly drop the cyclohexane solution of carbon tetrabromide into the three-necked flask. After dropping, the temperature was controlled at 25-30° C., and the reaction was stirred for 8 hours. Filter the reaction solution, wash the filter cake three times with n-hexane, combine the filtrates, remove the solvent by rotary evaporation, and then distill under reduced pressure (b.p.78-82°C, 1mmHg) to obtain 9.2g of 3-bromocyclohex-2-ene-1 Ketone, colorless liquid, yield 92%.
Embodiment 3
[0023] A kind of synthetic method of 3-bromocyclohex-2-en-1-one, comprises the steps:
[0024] Dissolve 53.2g (3.0eq.) of carbon tetrabromide in 15ml of cyclohexane; take a 250mL three-necked flask, add 100mL of toluene, and stir to dissolve 6.0g (1.0eq.) of 1,3-cyclohexanedione Add 56.0g (4.0eq.) triphenylphosphine into the three-necked flask, replace the argon protection, heat the water bath to 60-70°C, and slowly add the cyclohexane solution of carbon tetrabromide into the three-necked flask; After completion, control the temperature at 45-60°C, stir and react for 3 hours; after the reaction is completed, cool down to room temperature, filter the reaction solution, wash the filter cake with n-hexane for 3 times, combine the filtrate, remove the solvent by rotary evaporation, and then distill under reduced pressure (b.p.78-82°C, 1 mmHg) 8.9 g of 3-bromocyclohex-2-en-1-one was obtained as a colorless liquid, yield 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com