Formic acid cleavage site peptides and their related biomaterials and their application in the production of calcitonin
A formic acid cutting, biological material technology, applied in the direction of calcitonin, application, plant products, etc., can solve the problems of difficult mass production, high market price of calcitonin, low biological activity and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Example 1. Screening of Asp-Pro formic acid cleavage site peptides suitable for Asp-Pro formic acid cleavage method (formic acid cleavage method of peptide bond D-P (Asp-Pro))
[0090] In this example, four kinds of Asp-Pro formic acid cleavage site peptides in Table 1 are designed, and the names are CA1, CA2, CA3 and CA4 respectively. Asp-Pro formic acid cleavage site peptide CA3 with high cleavage efficiency was screened among the formic acid cleavage site peptides.
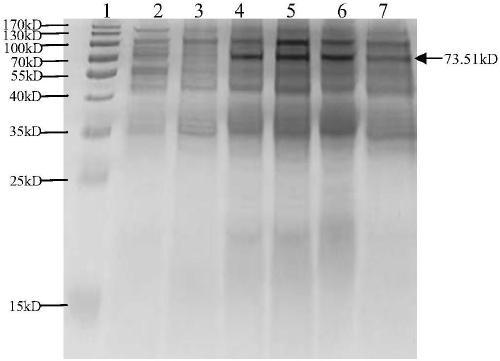
[0091] The four fusion proteins FP1, FP2, FP3 and FP4 were obtained by fusing salmon calcitonin with the extramembrane region of the glycoprotein through the four Asp-Pro formic acid cleavage site peptides, and the Asp-Pro formic acid cleavage site peptide in FP1 It is CA1, the Asp-Pro formic acid cleavage site peptide in FP2 is CA2, the Asp-Pro formic acid cleavage site peptide in FP3 is CA3 (amino acid sequence such as SEQ ID No.2), the Asp-Pro formic acid cleavage site in FP4 The peptide is CA4. The...
Embodiment 2
[0128] Example 2. Using Asp-Pro Formic Acid Cleavage Site Peptide CA3 to Cultivate Salmon Calcitonin Transgenic Rapeseed
[0129] 2.1 Construction of fusion protein G-sCT-SO gene expression vector
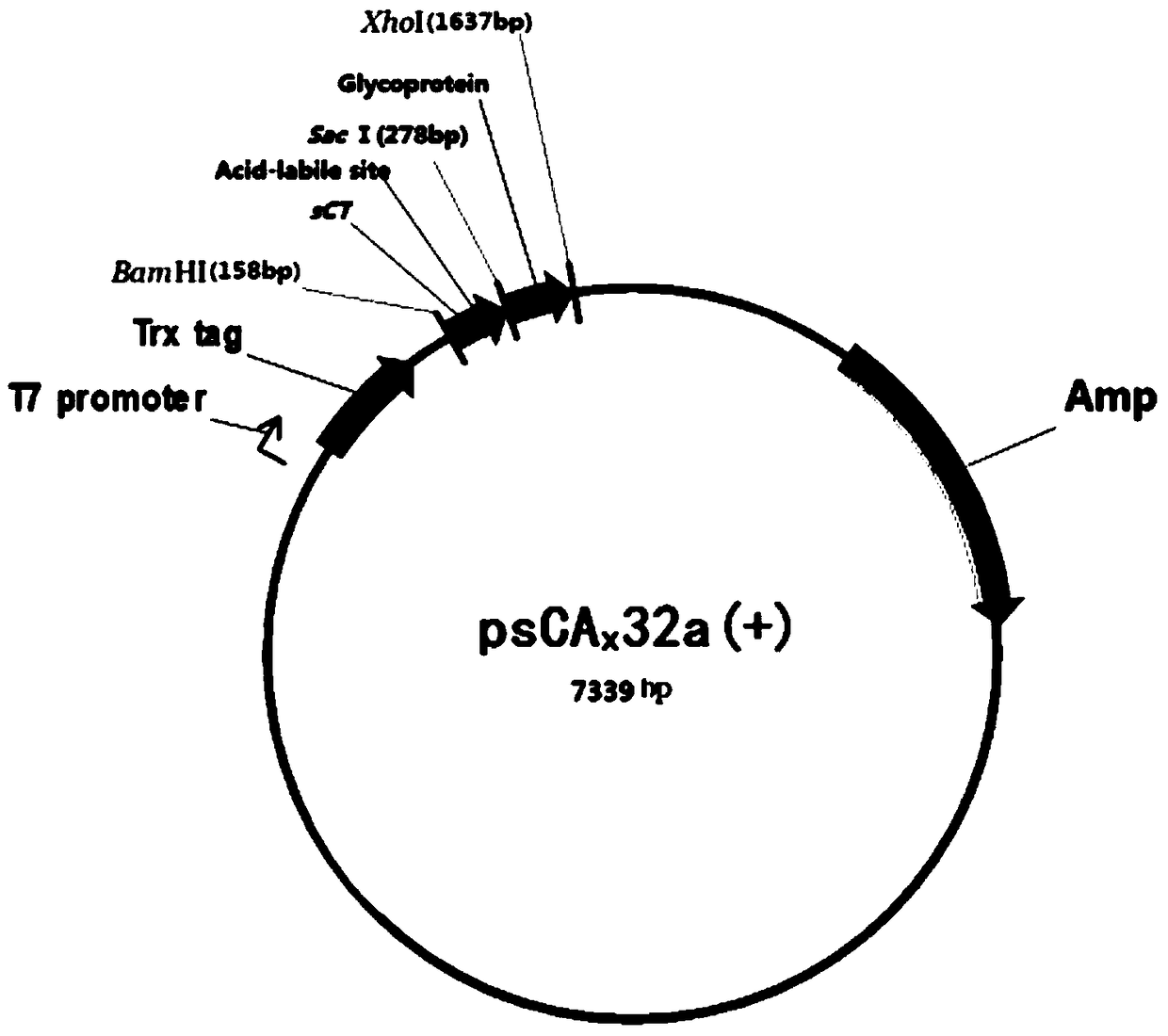
[0130] The fusion protein G-sCT was obtained by linking salmon calcitonin with the extramembrane region of the glycoprotein through the Asp-Pro formic acid cleavage site peptide CA3. In order to express the fusion protein G-sCT in the oil body of rapeseed, the fusion protein G- One end of sCT was linked with sesame oleosin to obtain fusion protein G-sCT-SO. The amino acid sequence of the fusion protein G-sCT-SO is SEQ ID No.4, in SEQ ID No.4, the 8th-150th is the sequence of the sesame oil body protein, the 153-208th is the sequence of the extramembrane region of the glycoprotein, and the 1st Positions 209-214 are the sequence of Asp-Pro formic acid cleavage site peptide CA3, and positions 215-247 are the sequence of salmon calcitonin.
[0131] Artificially synthesized fusion pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com