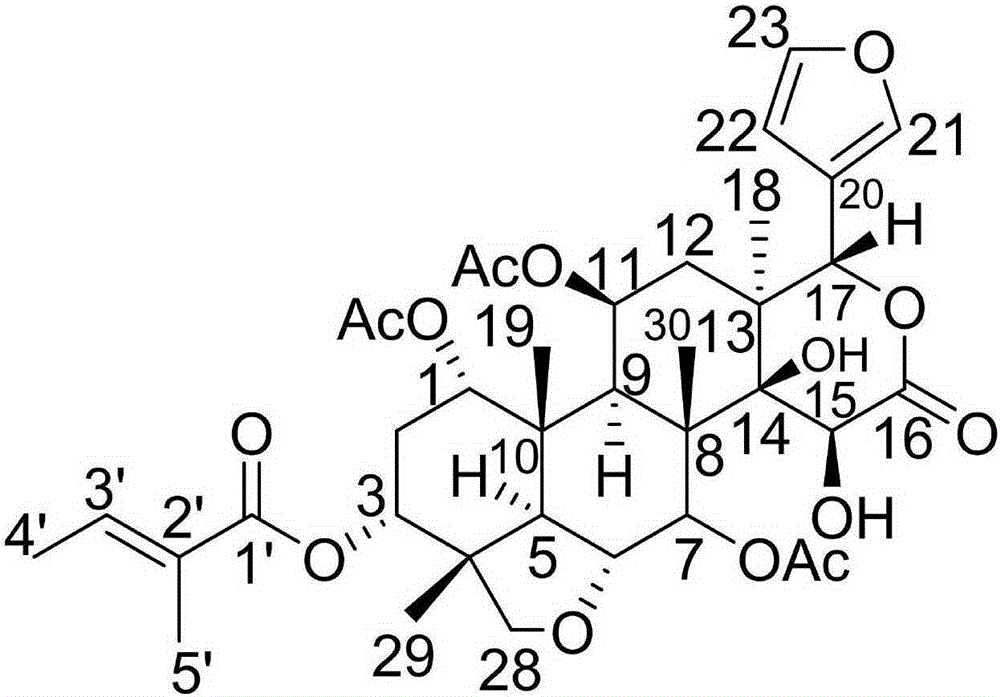
Novel limonin compound, preparation method therefor and medical application thereof
A compound and drug technology, applied in the field of limonoid compounds, can solve the problems of reduced content, no obvious correlation between content differences and varieties and origins, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
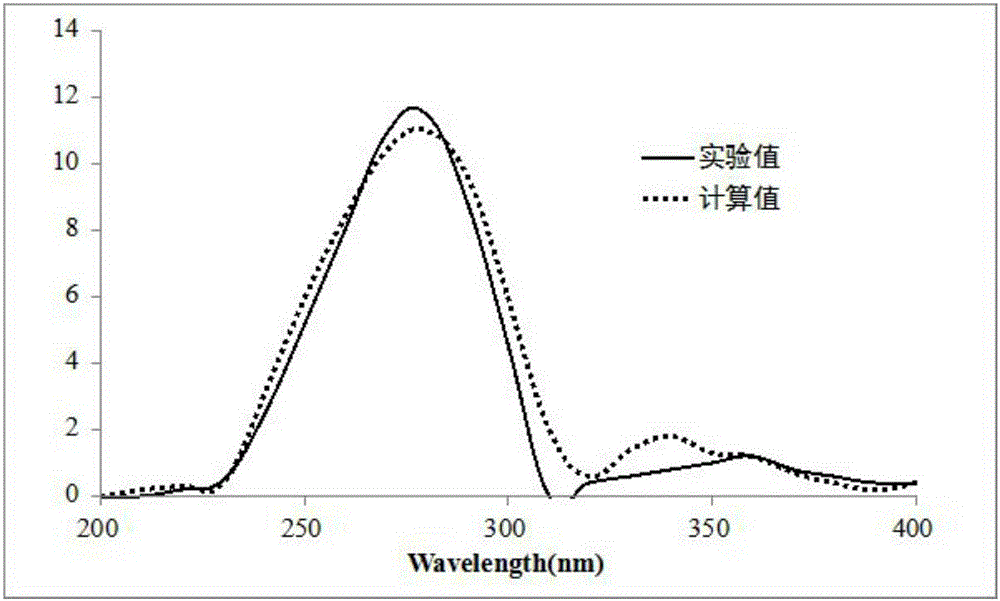
[0026] Example 1: Compound (I) Separation Preparation and Structure Confirmation
[0027] (a) The dried young fruit (10kg) of Aurantium citrifolia is crushed, extracted with 70% ethanol under heat reflux (30L×3 times), the extracts are combined, concentrated to no alcohol smell (6L), and then extracted with petroleum ether (6L×3 times) ), ethyl acetate (6L×3 times) and water-saturated n-butanol (6L×3 times) were extracted to obtain petroleum ether extract, ethyl acetate extract (375g) and n-butanol extract respectively; (b) In the step (a), the ethyl acetate extract was removed with D101 macroporous resin, first eluted with 10% ethanol for 6 column volumes, then with 70% ethanol for 8 column volumes, collected 70% eluent, and reduced Concentrate under pressure to obtain 70% ethanol elution concentrate (125g); (c) in step (b), 70% ethanol elution concentrate is separated with normal phase silica gel, and the volume ratio is 40:1 (8 column volumes), 20:1 (8 column volumes), 10:...
Embodiment 2
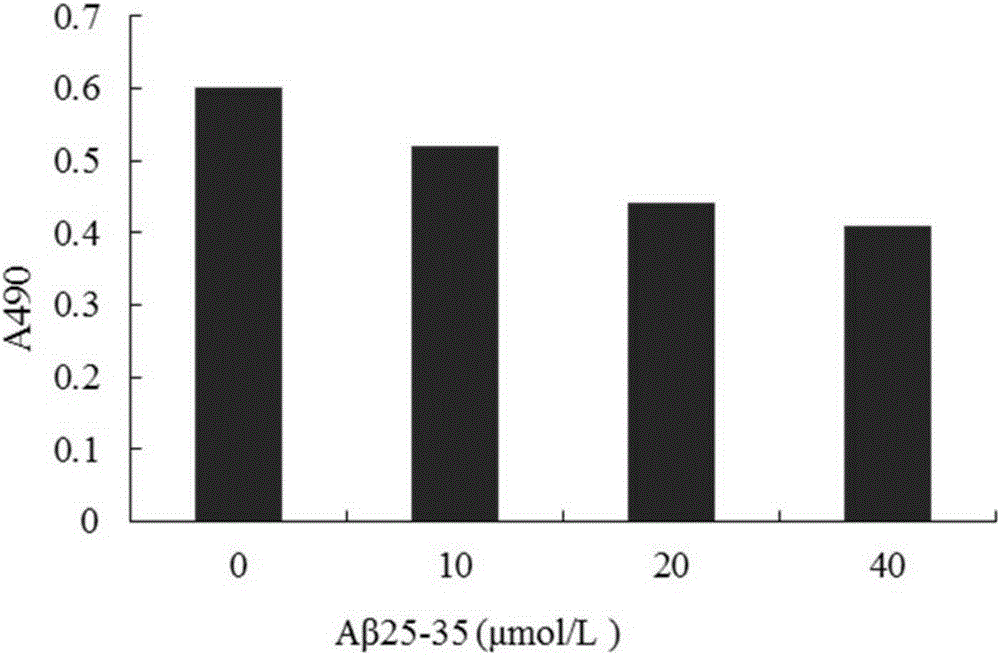
[0029] Embodiment 2: compound (I) pharmacological action test
[0030] 1. Materials and Instruments
[0031] Aβ 25-35 Purchased from sigma company in the United States. Compound (I) is self-made, the preparation method is shown in Example 1, and the HPLC normalized purity is greater than 98%. MTT (nitroblue tetrazolium) was purchased from Amresco, USA. LDH assay kit was purchased from Nanjing Jiancheng Biological Co., Ltd. Acridine orange (AO) was purchased from Sigma, USA. Ethidium bromide (EB) was purchased from Sigma, USA. RNase enzyme was purchased from Sigma, USA. Proteinase K was purchased from sigmaAnnexin, USA. V-FITC cell apoptosis detection kit was purchased from Nanjing KGI Biotechnology Development Co., Ltd. The dry powder of D-MEM / F12 medium was purchased from GibcoL, USA. Horse serum was purchased from hycLon Company in the United States. Fetal bovine serum was purchased from Hangzhou Sijiqing Biological Engineering Co., Ltd. Polylysine (PLL) was purch...
Embodiment 3
[0066] Preparation of tablet: firstly prepare compound (I) according to the method of Example 1, and utilize organic acids such as tartaric acid, or citric acid, formic acid or oxalic acid, etc., inorganic acids such as hydrochloric acid or sulfuric acid or phosphoric acid to make salts, according to its The weight ratio of the excipient is 1:7, and the excipient is granulated and compressed into tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of unsaturation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com