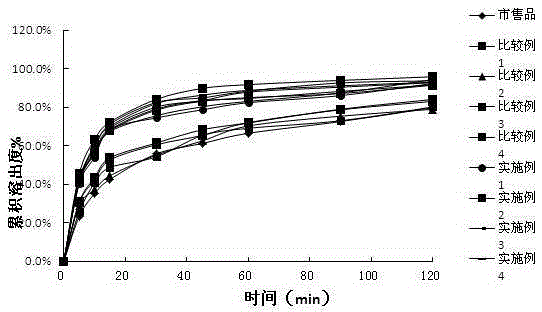
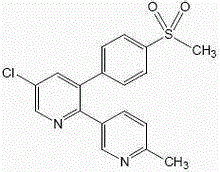
Drug combination containing etoricoxib and preparation method thereof
A technology of etoricoxib and composition, which is applied in the field of pharmaceutical compositions containing etoricoxib and its preparation, can solve the problems such as inability to effectively guarantee tablet hardness, low drug dissolution rate, and reduced drug dissolution rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Prescription composition:
[0031] Name of raw material Percentage (%w / w) Amount of raw and auxiliary materials (g) etoricoxib 25.0% 50.0 Calcium hydrogen phosphate anhydrous 35.0% 70.0 microcrystalline cellulose 35.0% 70.0 Croscarmellose sodium (internal) 1.5% 3.0 Sodium carboxymethyl starch (inside) 0.5% 1.0 Magnesium stearate (inside) 0.5% 1.0 Croscarmellose sodium (external) 1.5% 3.0 Sodium carboxymethyl starch (external) 0.5% 1.0 Magnesium stearate (external) 0.5% 1.0 made into tablets 200mg / tablet Makes 1000 pieces
[0032] Preparation:
[0033] a) Weighing: Mix 50g etoricoxib, 70g anhydrous calcium hydrogen phosphate, 3g croscarmellose sodium, 1g carboxymethyl starch sodium and 70g microcrystalline cellulose, then add 1g stearin Magnesium acid mixed to obtain mixture I;
[0034] b) Granulation: Mixture I is granulated with a pressure roller dry granulator to obtain Mixtur...
Embodiment 2
[0039] Prescription composition:
[0040] Name of raw material Percentage (%w / w) Amount of raw and auxiliary materials (g) etoricoxib 30.0% 60.0 Calcium hydrogen phosphate anhydrous 16.5% 33.0 microcrystalline cellulose 49.5% 99.0 Croscarmellose sodium (internal) 1.125% 2.25 Carboxymethyl starch press (internal) 0.375% 0.75 Magnesium stearate (inside) 0.5% 1.0 Croscarmellose sodium (external) 1.125% 2.25 Carboxymethyl starch press (external) 0.375% 0.75 Magnesium stearate (external) 0.5% 1.0 made into tablets 200mg / tablet Makes 1000 pieces
[0041] Preparation:
[0042] a) Weighing: mix 60g etoricoxib, 33g anhydrous calcium hydrogen phosphate, 2.25g croscarmellose sodium, 0.75g carboxymethyl starch sodium and 99g microcrystalline cellulose, then add 1g Magnesium stearate is mixed to obtain mixture I;
[0043] b) Granulation: Mixture I is granulated with a pressure roller dry granulator...
Embodiment 3
[0048] Prescription composition:
[0049] Name of raw material Percentage (%w / w) Amount of raw and auxiliary materials (g) etoricoxib 40.0% 80.0 Calcium hydrogen phosphate anhydrous 42.0% 84.0 microcrystalline cellulose 14.0% 28.0 Croscarmellose sodium (internal) 0.975% 1.95 Sodium carboxymethyl starch (inside) 0.325% 0.65 Magnesium stearate (inside) 0.5% 1.0 Croscarmellose sodium (external) 0.975% 1.95 What about carboxymethyl starch (external) 0.325% 0.65 Magnesium stearate (external) 0.5% 1.0 made into tablets 200mg / tablet Makes 1000 pieces
[0050] Preparation:
[0051] a) Weighing: Mix 80g etoricoxib, 84g anhydrous calcium hydrogen phosphate, 2.25g croscarmellose sodium, 0.75g carboxymethyl starch sodium and 28g microcrystalline cellulose, then add 1g hardened Magnesium fatty acid is mixed to obtain mixture I;
[0052] b) Granulation: Mixture I is granulated with a pressure roller...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com