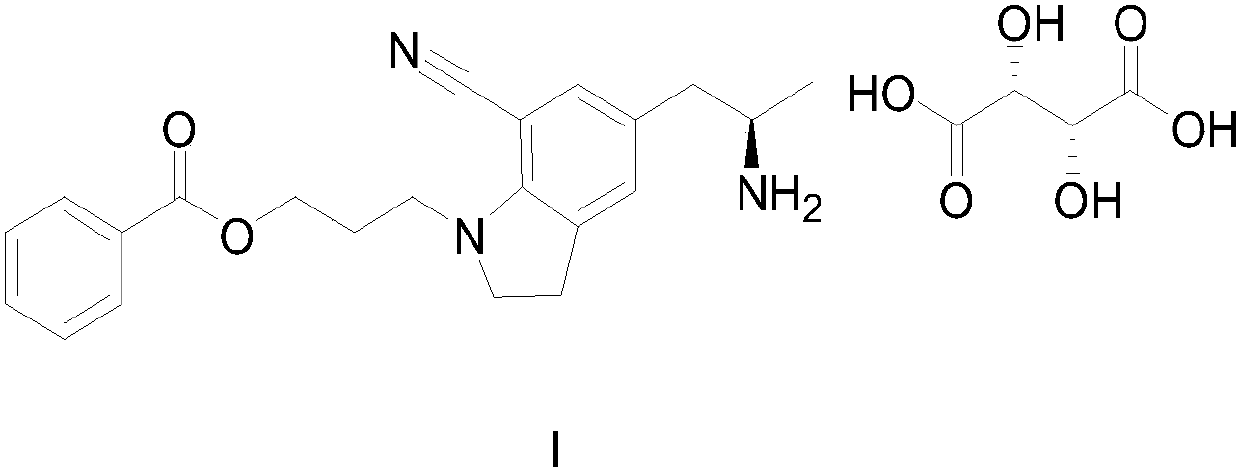
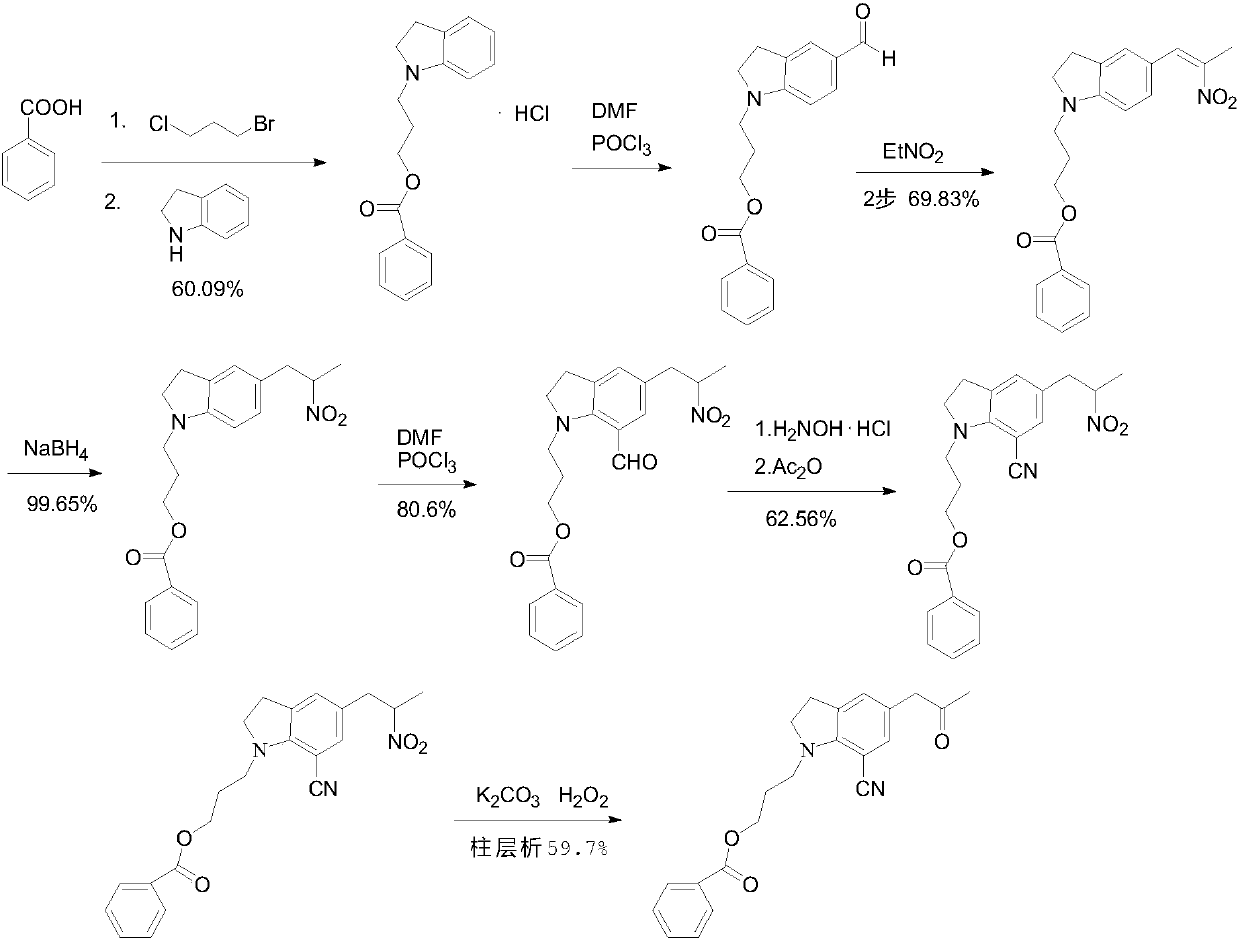
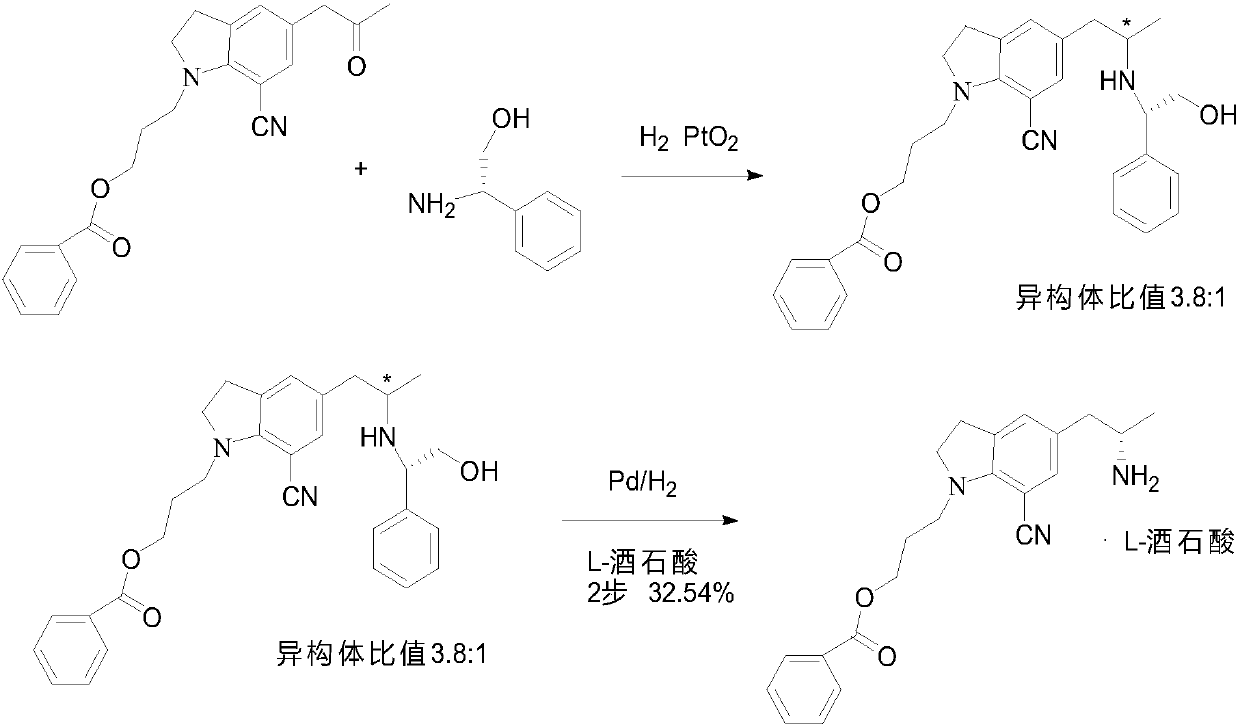
The preparation method of silodosin intermediate
A technology of intermediates and compounds, applied in the preparation of carboxylate, bulk chemical production, organic chemistry, etc., can solve the problems of high cost, complicated preparation process, expensive chiral reagents, etc., to reduce production costs and simplify the synthesis scheme easy to control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: the preparation of compound (4-1)
[0057]
[0058] Add 300 milliliters of anhydrous dichloromethane into the reactor, add 60.0 grams of anhydrous aluminum trichloride under stirring, after adding, cool to room temperature in a water bath, add 47.6 grams of 1-(3-benzoyloxypropyl)ind Indoline hydrochloride, stirred for 10 minutes, nitrogen protection. 52.4 g of (R)-2-acetamidopropionyl chloride was dissolved in dichloromethane solution and added dropwise to the above mixed solution. After dropping, raise the temperature to reflux to continue the reaction. After the reaction of the raw materials is complete, the reaction solution is lowered to room temperature, added to 3000 ml of ice-water mixture, stirred, added with sodium bicarbonate to adjust the pH to 7, allowed to stand for stratification, and dichloromethane layer, the upper aqueous layer was extracted twice with 250 ml of dichloromethane, the combined dichloromethane layers were washed with satu...
Embodiment 2
[0059] Embodiment 2: the preparation of compound (5-1)
[0060]
[0061] Add 39.4 grams of (4-1), 5.8 grams of trifluoroacetic acid and 150 milliliters of toluene into the reaction vessel, stir and add 25.5 grams of triethylsilane, nitrogen protection, react at 45 ° C for 22 hours, the raw materials basically react completely, and the reaction solution Cool down to room temperature, add the reaction solution to 300 ml of ice-water mixture, stir for half an hour, let stand to separate layers, extract the water layer twice with 100 ml of toluene, combine the organic layers, wash with saturated sodium bicarbonate solution, and wash with saturated brine, After drying, the desiccant was filtered out, and the filtrate was evaporated to dryness under reduced pressure to obtain a dark reddish-brown oil, which was obtained by column chromatography, which was compound (5-1).
Embodiment 3
[0062] Embodiment 3: the preparation of compound (6-1)
[0063]
[0064] Under ice-water cooling, 30.7 grams of phosphorus oxychloride was slowly added dropwise to 40.8 grams of DMF. After the drop, the ice-water bath continued to stir for half an hour. Under nitrogen protection, 38.0 grams of compound (5-1) was dissolved in Add 40ml of dichloromethane dropwise to the reaction solution, control the reaction temperature at 45-50°C, stir at 50°C for three hours after the drop is complete, and slowly add dropwise into 350ml of ice-water mixture after the reaction solution is cooled to room temperature , stirred for half an hour, extracted three times with 300 ml of dichloromethane, combined the organic layers, washed with saturated sodium bicarbonate solution, washed with saturated saline, dried, filtered out the desiccant, and the filtrate was evaporated to dryness under reduced pressure to obtain a reddish-brown oily substance, namely Compound (6-1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com