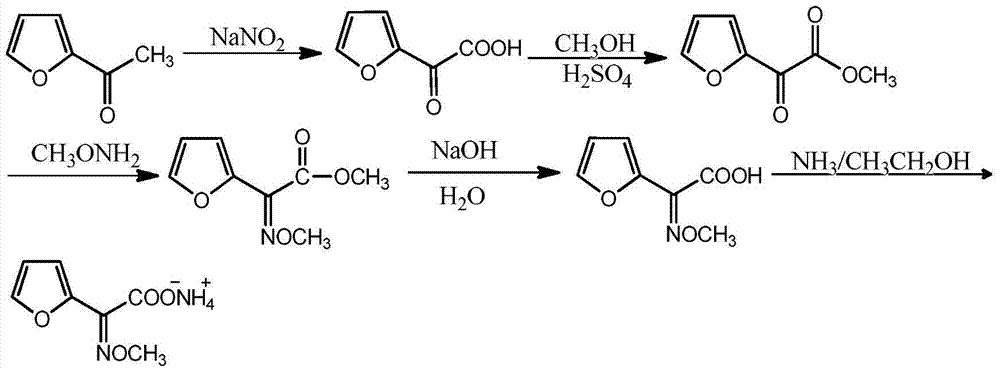
Synthetic process of furan ammonium salt
A technology of furan ammonium salt and synthesis process, applied in the direction of organic chemistry, etc., can solve the problem of low conversion rate of furanone acid, achieve the effects of improving yield and purity, reducing production cost, and reducing content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Mix and stir 100mL water and 150g 2-acetylfuran, control the temperature at 30°C, slowly add sodium nitrite aqueous solution (prepared by mixing 113g sodium nitrite and 153mL water) dropwise, after the dropwise addition, add concentrated hydrochloric acid ( The mass fraction is 36%) to adjust the pH value to 2.0 to obtain a furanonic acid solution, and the liquid phase detection shows that the conversion rate is 97.5%;
[0028] (2) Add 300 g of anhydrous methanol to the obtained furanone acid solution, and control the temperature of the solution to 35 ° C. At the same time, add 3 drops of concentrated sulfuric acid to the mixed solution, and react for 2.0 h under stirring. After the reaction is completed, distill the reaction solution , remove excess methyl alcohol, obtain furanonic acid methyl ester;
[0029] (3) Lower the temperature to 20°C, add 110 g of methoxyamine dropwise to the obtained methyl furanone, adjust the pH to 2.0, react for 1.5 hours, and detect b...
Embodiment 2
[0033] (1) Mix and stir 200mL of water and 306g of 2-acetylfuran, control the temperature at 0°C, slowly add an aqueous solution of sodium nitrite (prepared by mixing 282g of sodium nitrite and 312mL of water), and after the addition is complete, add concentrated hydrochloric acid ( The mass fraction is 36%) to adjust the pH value to 3.0 to obtain a furanonic acid solution, and the liquid phase detection shows that the conversion rate is 98.7%;
[0034] (2) Add 650g of absolute ethanol to the obtained furanone acid solution and control the temperature of the solution to 37°C. At the same time, add 3 drops of concentrated sulfuric acid to the mixed solution, and react for 1.2h under stirring. After the reaction is completed, distill the reaction solution , remove excess ethanol, obtain ethyl furanone;
[0035] (3) Lower the temperature to 30°C, add 230 g of methoxyamine dropwise to the obtained ethyl furanone, adjust the pH to 4.0, react for 2.6 hours, detect in liquid phase, t...
Embodiment 3
[0039] (1) Mix and stir 50mL water and 40g 2-acetylfuran, control the temperature at 78°C, slowly add sodium nitrite aqueous solution (prepared by mixing 50g sodium nitrite and 68mL water) dropwise, after the dropwise addition, add concentrated hydrochloric acid ( The mass fraction is 36%) to adjust the pH value to 2.0 to obtain a furanonic acid solution, and the liquid phase detection shows that the conversion rate is 96.1%;
[0040] (2) Add 90g of anhydrous methanol to the obtained furanone acid solution and control the temperature of the solution to be 32°C. At the same time, add 3 drops of concentrated sulfuric acid dropwise to the mixed solution and react for 5h under stirring. After the reaction is completed, distill the reaction solution to remove Excessive methyl alcohol, obtains methyl furanone;
[0041] (3) Lower the temperature to 30°C, add 48 g of methoxyamine dropwise to the obtained methyl furanone, adjust the pH to 4.0, react for 2.6 hours, detect in liquid phas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com