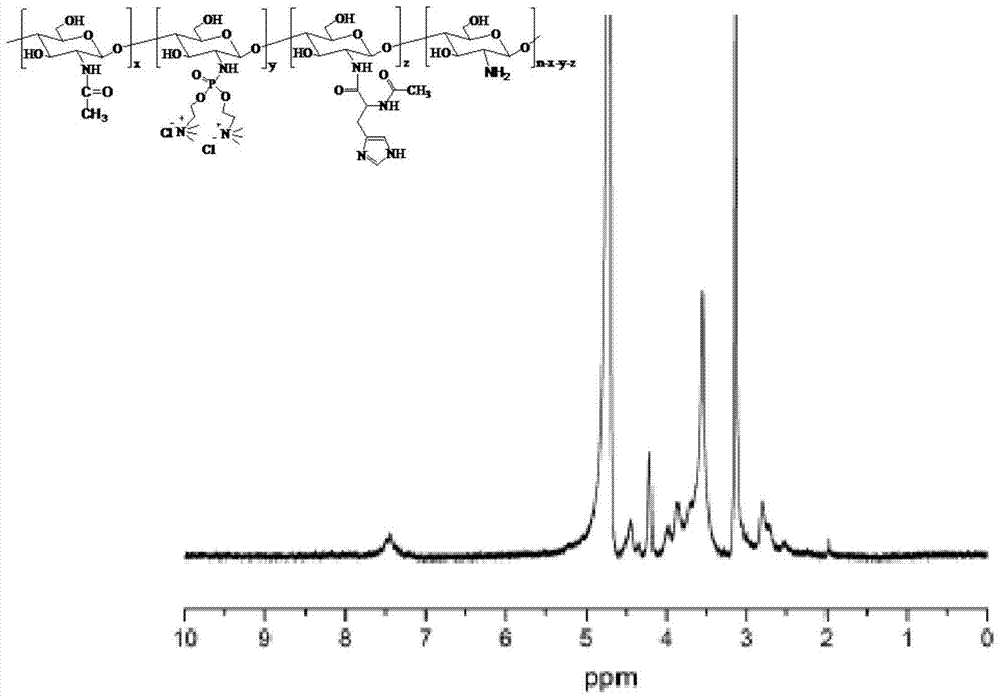
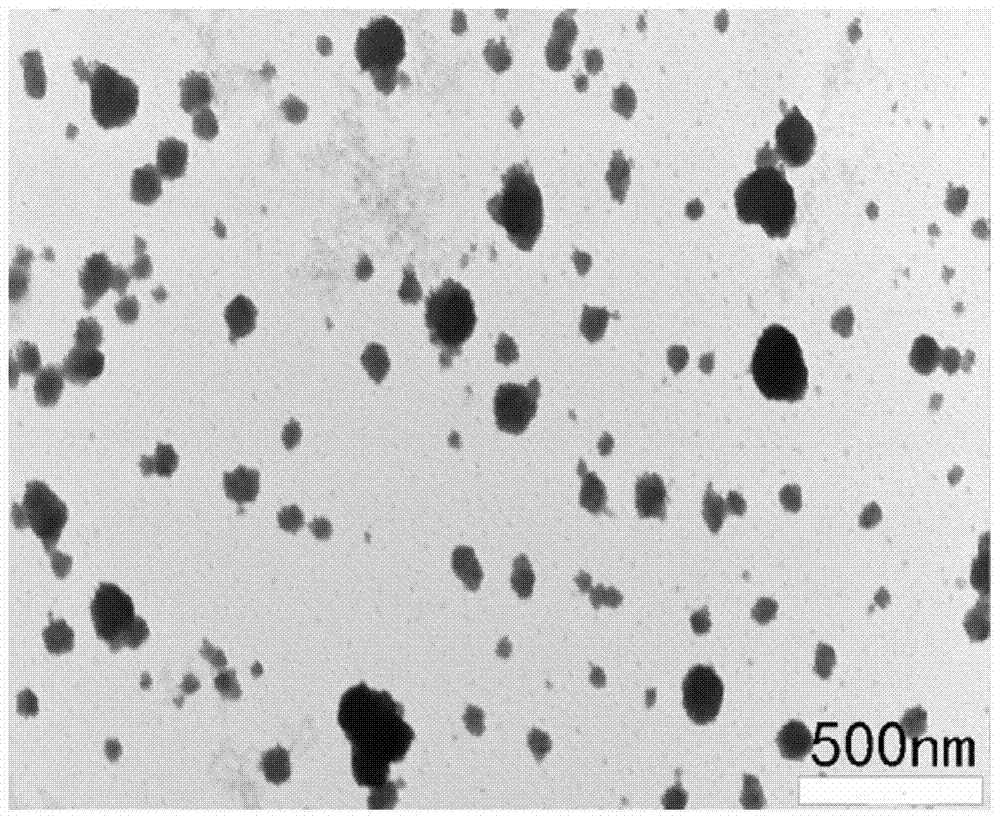
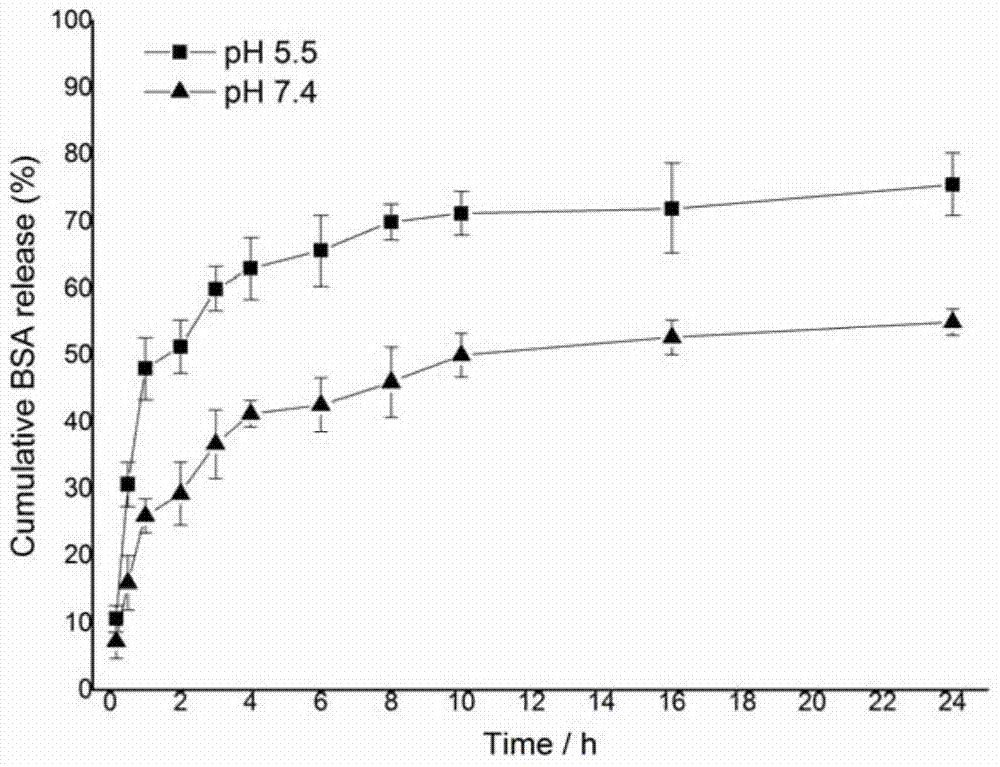
A cationic chitosan biomimetic derivative and its application
A cationic chitosan technology, applied in medical preparations with non-active ingredients, medical preparations containing active ingredients, peptide/protein ingredients, etc., can solve the problem of affecting protein activity, low cell entry efficiency, and high toxicity and other issues, to achieve the effect of maintaining, improving intracellular transmission efficiency, and promoting escape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 The synthesis of acetylhistidine-phosphate dicholine chitosan hydrochloride
[0034] 1. Dissolve 200 mg of 6-O-triphenylmethyl etherified chitosan (CsTr) modified by chitosan (x / n=0) in 10 mL of anhydrous dimethylacetamide, and add 0.42 mL of triethylamine and 0.19 mL of carbon tetrachloride; slowly add 0.76 g of disubstituted choline phosphonate, wherein the molar ratio of the amino group in CsTr to phosphonate is 1:4, and stir for 10 hours; spin to dry the solvent , add formic acid, stir at room temperature for 2 hours; spin dry formic acid, dialyze with physiological saline and deionized water, and freeze-dry to obtain phosphoric acid dicholine chitosan hydrochloride.
[0035] 2. Dissolve 520mg (2.6mmol) of acetyl histidine in 20mL of anhydrous DMSO, remove a small amount of water by rotary evaporation, add 1.62g (10mmol) of N,N-carbonyldiimidazole (CDI), stir at room temperature for 4 hours, and rotate to evaporate Remove DMSO, then add 100 mg phosphor...
Embodiment 2
[0036] Embodiment 2 The synthesis of acetylhistidine-phosphate dicholine chitosan hydrochloride
[0037] 1. Dissolve 500mg of 6-O-triphenylmethyl etherified chitosan (CsTr) modified by chitosan (x / n=0.2) in 40mL of anhydrous dimethylacetamide, and add 1.05mL of triethylamine and 0.49 mL of carbon tetrachloride; slowly add 2.3 g of disubstituted choline phosphonate, wherein the molar ratio of the amino group in CsTr to phosphonate is 1:6, and stir for 12 hours; spin to dry the solvent , add formic acid, stir at room temperature for 3 hours; spin dry formic acid, dialyze with normal saline and deionized water for 3 days, and freeze-dry to obtain phosphoric acid dicholine chitosan hydrochloride.
[0038] 2. Dissolve 260mg (1.3mmol) of acetyl histidine in 15mL of anhydrous DMSO, remove a small amount of water by rotary evaporation, add 1.62g (10mmol) of N,N-carbonyldiimidazole (CDI), stir at room temperature for 12 hours, and rotate to evaporate Remove DMSO, then add 100 mg phosp...
Embodiment 3
[0039]Example 3 Synthesis of acetylhistidine-phosphate dicholine chitosan hydrochloride
[0040] 1. Dissolve 300mg of 6-O-triphenylmethyl etherified chitosan (CsTr) modified by chitosan (x / n=0.1) into 30mL of anhydrous dimethylacetamide, and add 0.63mL triethylamine and 0.29 mL of carbon tetrachloride; slowly add 1.84 g of disubstituted choline phosphonate, wherein the molar ratio of the amino group in CsTr to phosphonate is 1:8, and stir for 12 hours; spin to dry the solvent , add formic acid, stir at room temperature for 6 hours; spin dry formic acid, dialyze with normal saline and deionized water for 3 days, and freeze-dry to obtain phosphoric acid dicholine chitosan hydrochloride.
[0041] 2. Dissolve 520mg (2.6mmol) of acetyl histidine in 20mL of anhydrous DMSO, remove a small amount of water by rotary evaporation, add 0.81g (5mmol) N,N-carbonyldiimidazole (CDI), stir at room temperature for 6h, and rotate to evaporate Remove DMSO, then add 100 mg phosphoric acid dicholi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com