Preparation method of whitmania pigra whitman extract sustained-release pellet
A technology of wide-body golden leech and slow-release pellets, which is applied in the direction of medical preparations, drug combinations, and pharmaceutical formulations of non-active ingredients, and can solve the problem of thrombin instability, low utilization, and difficult stability issues, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
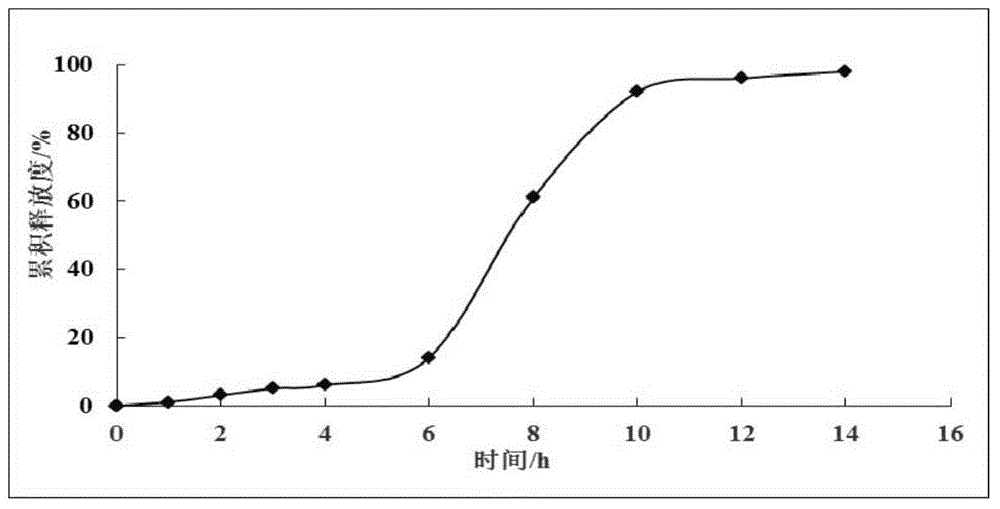
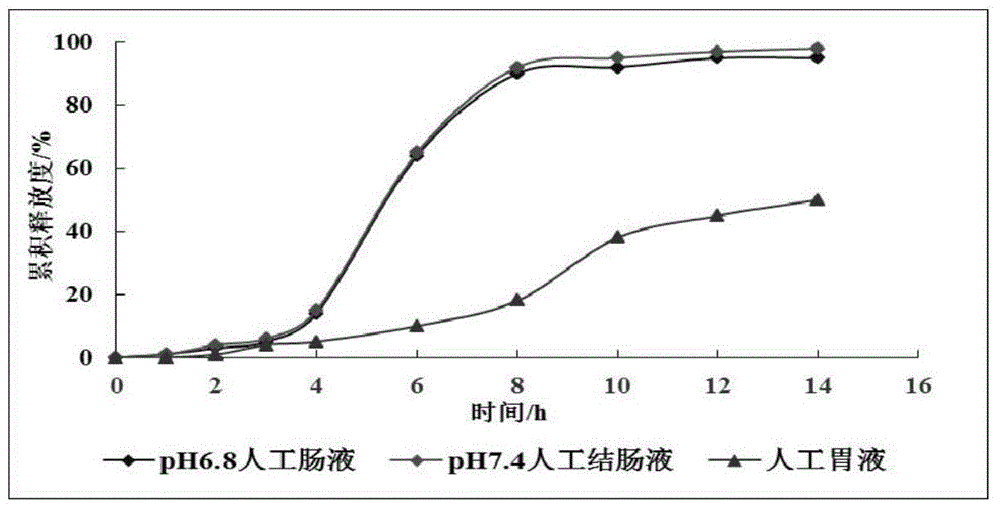
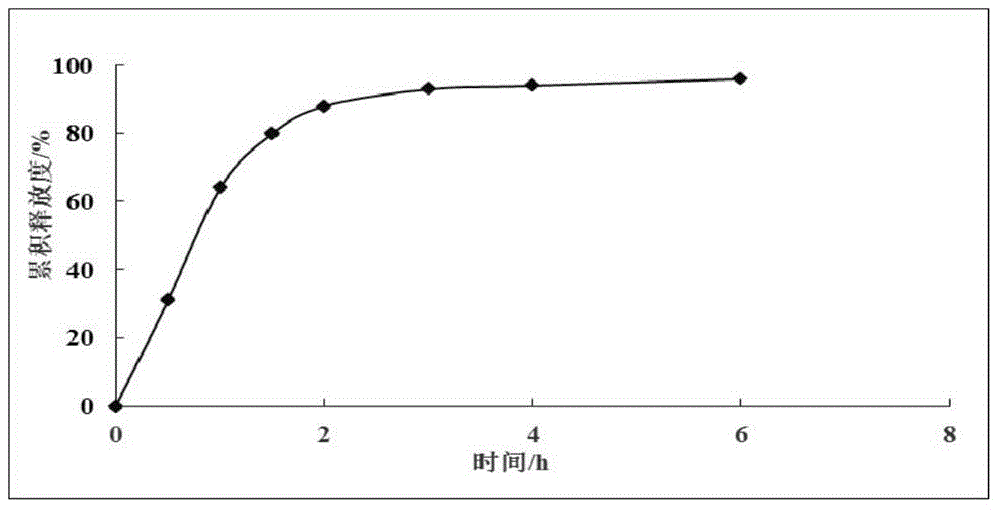
Image
Examples
Embodiment 1
[0029] Embodiment 1 (preparation of wide body leech extract and detection of protein yield and coagulation time)
[0030] 1. Physiological saline extraction
[0031] Take 100g of dry leeches wide body, crush it, add 1L of normal saline, soak it at 4°C for 4h, put it under the condition of 70°C, stir and extract it for 1h, then filter it, add 500ml of normal saline to the residue, and continue at 70°C Stirring and extracting for 1 h under the condition, the two extracts were combined and dried in vacuum.
[0032] 2. Distilled water extraction
[0033] Take 100g of dry leeches wide body, crush them, add 1L of distilled water, soak them at 4°C for 4h, place them at 70°C, stir and extract for 1h, then filter, add 500ml of distilled water to the residue, and continue to immerse at 70°C Stir and extract for 1 h, combine the two extracts, and dry in vacuum.
[0034] 3. Water extraction and alcohol precipitation
[0035] Take 100g of dry leeches wide body, crush it, add 10 times t...
Embodiment 2
[0047] 1. Preparation of Sustained-release Pellets of Broad-bodied Leech Extract
[0048] Drug-loaded pill core: get the broad-body leech extract 30g extracted by physiological saline described in Example 1, 62g microcrystalline cellulose, 2gHPMCE50, 5g lactose, 1g sodium caprate, make soft material with 100ml distilled water, in multiple The pellets were prepared on the functional pellet machine according to the following conditions: extrusion speed 28rpm, spheronization speed 560rpm, blast temperature 35°C, spheronization time: 5min, pellets dried at 40°C for 3h. Pass through a 24-40 mesh sieve to obtain drug-loaded pellets.
[0049] Swelling layer coating: Take 4g of polyvinylpyrrolidone and 16g of cross-linked polyvinylpyrrolidone and mix them evenly with 200ml of distilled water to obtain the swelling layer coating solution. The coating solution is atomized and coated on the surface of the drug-loaded pellets. Coating process: nozzle diameter 1mm, coating temperature 30...
Embodiment 3
[0056] 1. Preparation of Sustained-release Pellets of Broad-bodied Leech Extract
[0057] Drug-loaded pill core: get the broad-body leech extract 30g that physiological saline extracts described in embodiment 1, 62.4g microcrystalline cellulose, 2gHPMCE50, 5g lactose, 0.6gSANC make soft material with 100ml distilled water, in multifunctional micro The pellets were prepared on the pellet machine according to the following conditions: extrusion speed 28rpm, spheronization speed 560rpm, blast temperature 35°C, spheronization time: 5min, pellets were dried at 40°C for 3h. Pass through a 24-40 mesh sieve to obtain drug-loaded pellets.
[0058] Swelling layer coating: Take 4g of polyvinylpyrrolidone and 16g of cross-linked polyvinylpyrrolidone and mix them evenly with 200ml of distilled water to obtain the swelling layer coating solution. The coating solution is atomized and coated on the surface of the drug-loaded pellets. Coating process: nozzle diameter 1mm, coating temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com