Method for preparing epoxy propane from ethylbenzene hydroperoxide and propylene
A technology of ethylbenzene hydrogen peroxide and propylene oxide, which is applied in the direction of organic chemistry, can solve the problems of rising reaction temperature, achieve the effect of increasing reaction temperature, reducing the proportion of propylene circulation, and increasing the reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
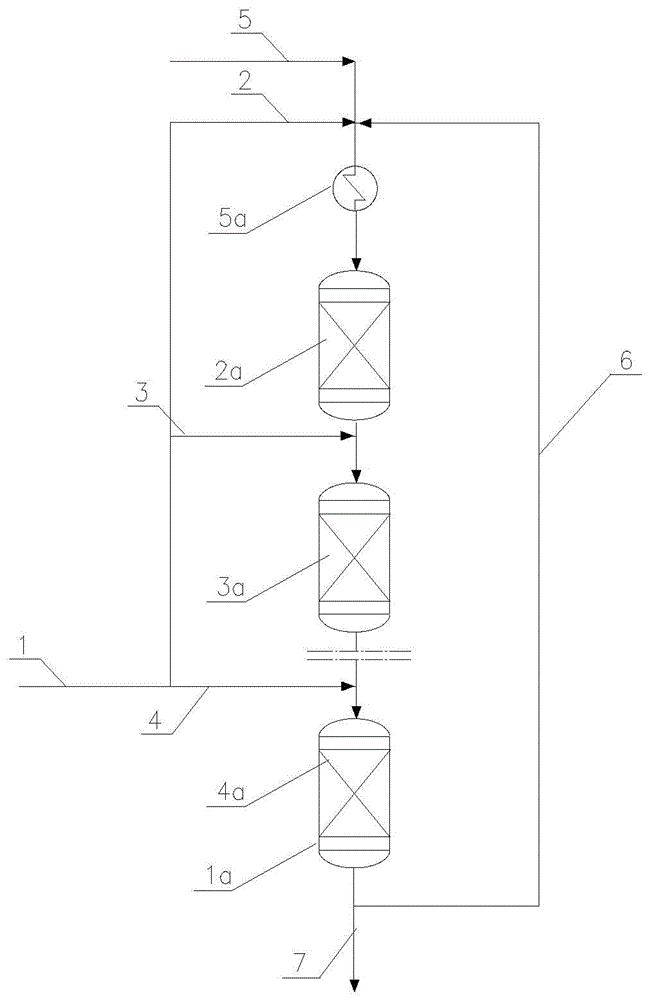
[0035] according to figure 1 In the technical scheme shown, the raw material I is a hydrogen peroxide ethylbenzene mixture containing 19.4% by weight, and the flow rate is 162367.5 kg / hour. The propylene flow rate in raw material II is 10987.6 kg / hour, the circulating propylene is 103863.5 kg / hour, the circulating flow rate in the epoxidation reaction product containing propylene oxide is 184812.6 kg / hour, and the hydrogen peroxide entering the first reactor catalyst bed Ethylbenzene mixture is mixed, under the conditions of reaction temperature 76°C and reaction pressure 2.9MPaG, contact with Ti / SiO2 catalyst, liquid phase epoxidation reaction to generate propylene oxide, methyl benzyl alcohol and other by-products; flow out of the first reaction The reactants in the catalyst bed of the second reactor are mixed with the ethylbenzene hydrogen peroxide mixture entering the catalyst bed of the second reactor, and the temperature is controlled at 76°C, and then enter the catalyst...
Embodiment 2
[0037] Same as [Example 1], only the flow rate of circulating propylene is changed: the circulating propylene flow rate is 69242.5 kg / hour, the circulating epoxidation reaction product is 161731.8 kg / hour, and others are the same as [Example 1]. Hydrogen peroxide ethylbenzene weight space velocity 4.1hr -1 , the mol ratio of total propylene to ethylbenzene hydroperoxide is 8.03, the mol ratio of propylene to ethylbenzene hydroperoxide at the inlet of the first catalyst bed and the second catalyst bed then reaches 30.17 and 21.11, the first catalyst bed and the outlet temperatures of the second catalyst bed were 87.0°C and 88.3°C respectively, the total conversion rate of ethylbenzene hydroperoxide was 99.2%, and the molar selectivity of propylene oxide was 99.1%.
Embodiment 3
[0039] Same as [Example 1], only the reactor form is changed: two double-bed adiabatic fixed-bed reactors are connected in series, and the others are the same as [Example 1]. Hydrogen peroxide ethylbenzene weight space velocity 4.1hr -1 , the mol ratio of total propylene and ethylbenzene hydrogen peroxide is 11.52, and the mol ratio of propylene and ethylbenzene hydrogen peroxide then reaches 43.95 and 31.07 at the catalyst bed inlet of the first reactor catalyst bed and the second reactor catalyst bed, the first The outlet temperatures of the catalyst bed of the first reactor and the catalyst bed of the second reactor were 85.2°C and 86.6°C respectively, the total conversion rate of ethylbenzene hydroperoxide was 99.2%, and the molar selectivity of propylene oxide was 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com