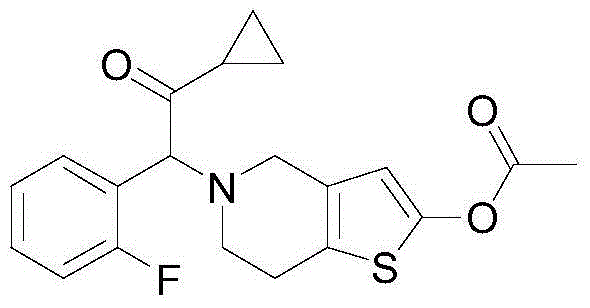
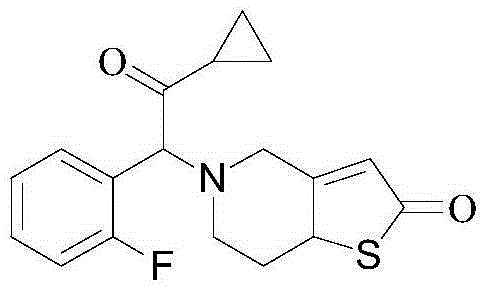
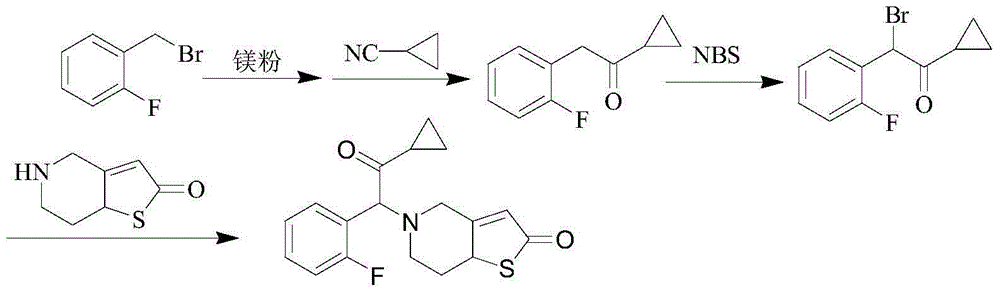
Method for preparing prasugrel intermediate
A technology for intermediates and compounds, applied in the field of organic chemistry, can solve problems such as being unsuitable for industrial production requirements, difficult to obtain raw materials, and expensive, and achieve the effects of significant industrial application value, easy large-scale production, and low production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the synthesis of compound 3
[0042]
[0043] Dissolve 35.7g (0.189mol) of compound 1 and 20g (0.157mol) of compound 2 in 100mL of tetrahydrofuran, add 20g (0.189mol) of sodium carbonate, and stir at room temperature for 2 hours; the reaction is complete, filter, and wash the filtrate with saturated saline , liquid separation, extract the aqueous phase with 20mL ethyl acetate, separate the liquid, combine the organic phases, dry with 10g of anhydrous sodium sulfate, filter, and distill under reduced pressure at 40°C. After the distillation is completed, 33.2g of yellow oily compound 3 is obtained, with a purity of 98.4 %, yield 89.7%.
[0044] 1 H-NMR (CDCl 3 ; TMS): δ: 2.33 ~ 2.67 (m, 4H, CH 2 CH 2 ), 3.81~3.93 (m, 2H, CH 2 ), 6.68~7.22 (m, 7H).
[0045] MS (ESI, m / z): 236 [M+H] + .
Embodiment 2
[0046] Embodiment 2: the synthesis of compound 4
[0047]
[0048] Dissolve 30g (0.121mol) of compound 3 in 150mL of methanol, add 4g (0.133mol) of paraformaldehyde, stir at room temperature until the reaction is complete (about 5 hours), add 10mL of concentrated hydrochloric acid, when TLC traces that the starting point disappears, stop Reaction, remove methanol by rotary evaporation, dissolve the residue with 150mL dichloromethane, wash with water, separate liquids, dry the organic phase with anhydrous sodium sulfate, filter, distill the filtrate under reduced pressure, evaporate, stir with 90mL methyl tert-butyl ether, filter , air-dried at 40° C. to constant weight to obtain 22.5 g of off-white solid compound 4 with a purity of 98.6% and a yield of 71.4%.
[0049] 1 HNMR (CDCl 3 ; TMS): δ: 2.58 ~ 2.64 (m, 4H, CH 2 CH 2 ), 3.52~3.59 (m, 2H, CH 2 ), 3.61~3.72 (m, 2H, CH 2 ), 6.33(s, 1H, CH), 6.46(s, 1H, CH), 7.12~7.52(m, 4H, ArH);
[0050] MS (ESI, m / z): 248 [M+H] ...
Embodiment 3
[0051] Embodiment 3: the synthesis of compound 5
[0052]
[0053]Dissolve 20g (0.081mol) of compound 4 in 100mL of methanol, add 9.8g (0.097mol) of triethylamine at the same time, cool down to 0-10°C, add dropwise a mixed solution of 12.9g (0.081mol) of bromine and 20mL of methanol, After dropping, react at room temperature for 2 hours. When TLC traces no raw material point, add 10% sodium thiosulfate aqueous solution dropwise, extract with 3×50mL dichloromethane, combine the organic phases, wash with 50mL water, separate the layers, and use Dry over sodium sulfate, filter, and distill the filtrate under reduced pressure to obtain a yellow oil, which is crystallized by stirring with 60 mL of methyl tert-butyl ether, filter, and air-dried at 40°C to obtain 19.3 g of compound 5 as a yellow solid, with a purity of 96.3%, yield 73.1%.
[0054] 1 HNMR (CDCl 3 ; TMS): δ: 2.42~2.61 (m, 4H, CH 2 CH 2 ), 3.58 (m, 2H, CH 2 ) 5.72 (s, 1H, CH), 6.19 (s, H, CH), 6.24 (s, H, CH), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com