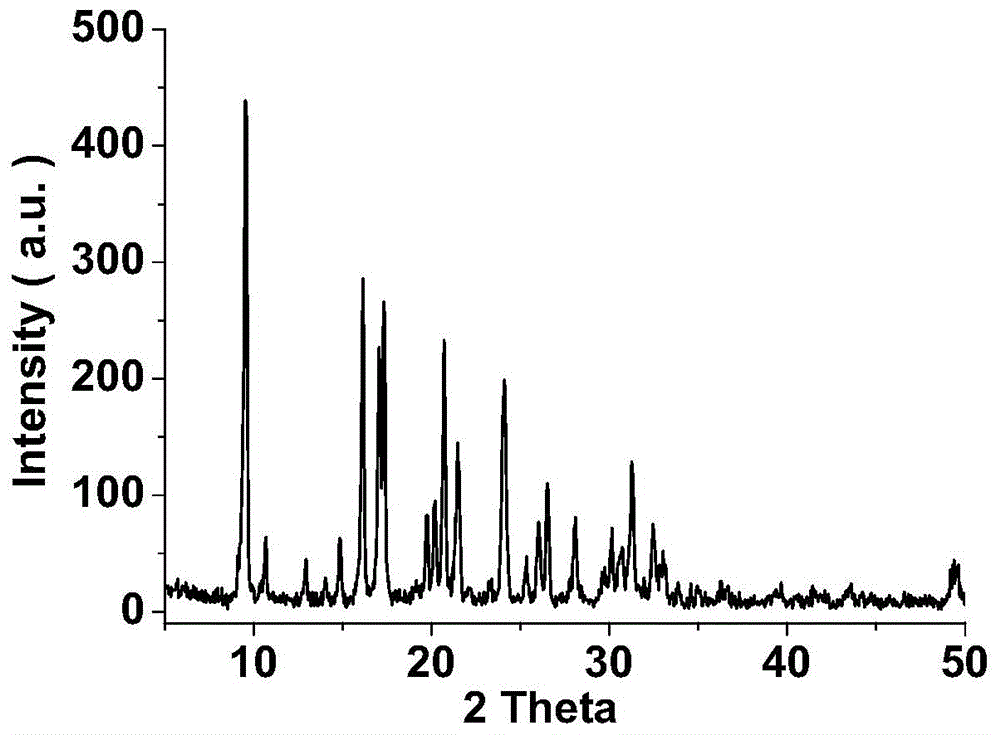
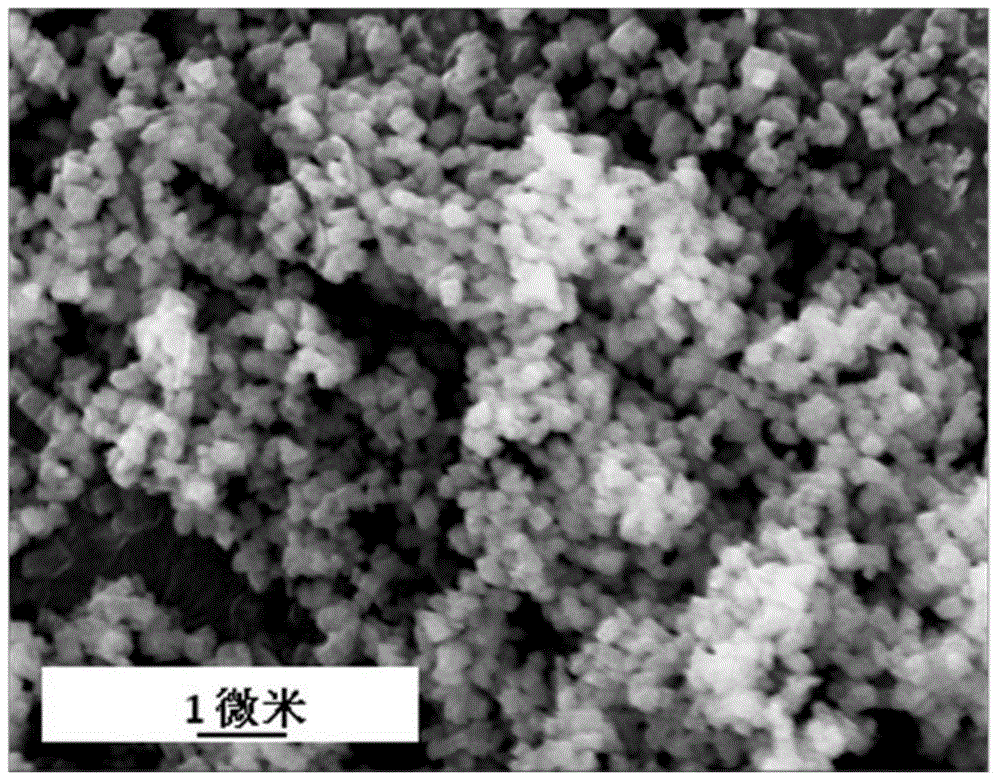
Template agent and preparation method therefor and application thereof
A technology of template agent and application, applied in the field of template agent and its preparation, can solve the problems of no effective preparation method, etc., and achieve the effect of controllable grain size, uniform distribution, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] In one embodiment of the present invention, the above-mentioned AEI type silica-alumina molecular sieve is prepared by the following preparation method, which includes the following steps:
[0056] 1) Colloidal preparation: mix template agent, alkali and water, then add Y-type molecular sieve and silicon source, stir evenly between 25-45°C;
[0057] 2) Get the sol of step 1), add 0.1-5wt% weight (relative to SiO in the silicon source 2 Weight) seed crystals are added to the autoclave, stirred, and crystallized at 130-200°C for 5-10 days, wherein the stirring speed is 80-240rpm.
[0058] In the present invention, the alkali is potassium hydroxide or sodium hydroxide.
[0059] In the present invention, the silicon source is selected from inorganic silicon. Preferably, it is silica sol containing silica or solid silica gel for column chromatography.
[0060] In the present invention, the Y-type molecular sieve is used as an aluminum source.
[0061] In the present inve...
preparation example 1
[0082] Preparation example 1 (preparation of template agent A)
[0083] Template agent A was prepared by the following steps:
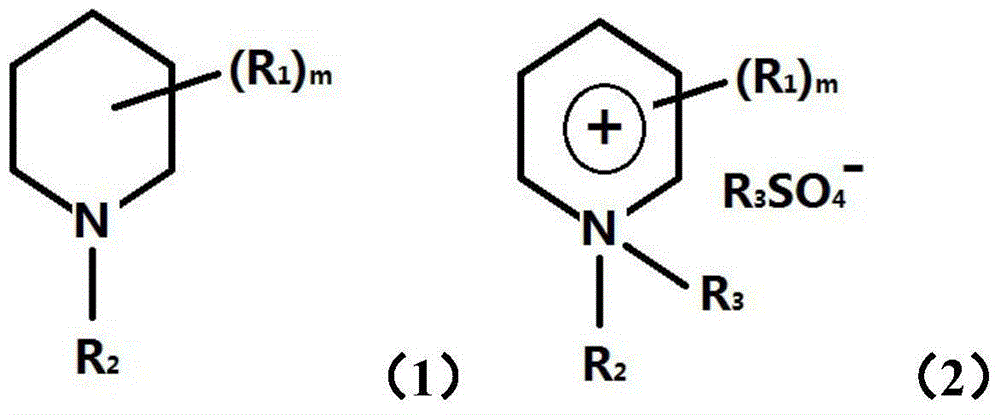
[0084] With 2,6-dimethyl-piperidine as the starting reactant, potassium hydroxide ethanol solution is added to its ethyl bromide ethanol solution, and the reaction occurs at room temperature to produce potassium bromide precipitation to generate 1-ethyl-2 , 6-Dimethyl-piperidine. Concentrate and evaporate to dryness to obtain product (molecular formula is C 9 h 19 N, recorded as template agent A).
[0085] This one-step reaction is a method known to those skilled in the art and will not be repeated here.
preparation example 2
[0086] Preparation example 2 (preparation of template B)
[0087] Template agent B was prepared by the following steps:
[0088] The 500ml toluene solution of the product template agent A (100g, 98%, 0.70 moles) of Preparation Example 1 and diethyl sulfate (180g, 98%, 1.14 moles) was reacted at 110°C in a 2L reflux vessel for 5-6 Hour, after cooling, 1,1-diethyl-2, the salt of 6-dimethyl-piperidinium monoethyl sulfate precipitates out from solution, filters and obtains white product 175.6g, yield 85% (molecular formula for C 13 h 29 NSO 4 , denoted as templating agent B).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com