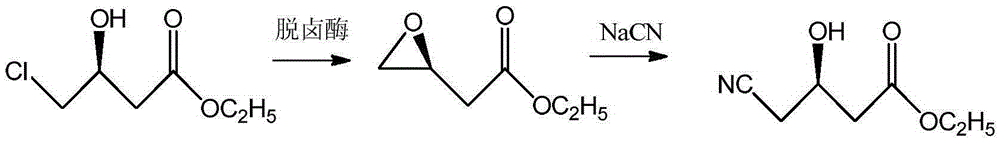
Process for enzymatic synthesis of (R)-4-cyano-3-hydroxybutyrate from sodium alginate immobilized halogenohydrin dehalogenase
A technology of ethyl hydroxybutyrate and halohydrin dehalogenase, applied in the field of medicine and chemical industry, can solve the problems of side reactions, many influencing factors, difficult to control, etc., and achieve the effects of high immobilization rate, high catalytic efficiency and fast immobilization rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A kind of technology that sodium alginate immobilizes halohydrin dehalogenase and catalyzes synthesis (R)-4-cyano-3-hydroxybutyric acid ethyl ester, comprises the following steps:
[0028] 1) Extract 15U / mL halohydrin dehalogenase solution from the crude halohydrin dehalogenase solution for use; prepare a sodium alginate aqueous solution with a mass fraction of 3.5wt% for use; prepare a volume fraction of 1% glutaraldehyde Aqueous solution ready for use;
[0029] 2) Drop 2 mL of glutaraldehyde aqueous solution into a uniformly mixed solution of 40 mL of sodium alginate aqueous solution and 40 mL of halohydrin dehalogenase solution, and react for 5 hours under stirring conditions to obtain a stationary solution;
[0030] 3) Drop the fixative solution into 100 mL of 2 wt % calcium chloride aqueous solution drop by drop to form bead beads, solidify for 1 hour and filter to obtain sodium alginate immobilized halohydrin dehalogenase;
[0031] 4) Weigh 207g of distilled wate...
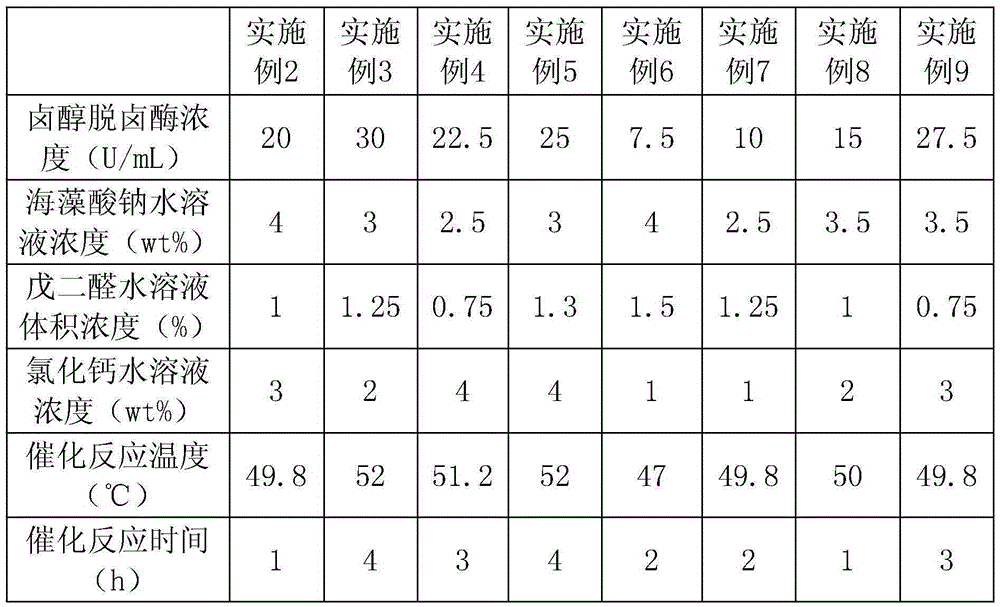
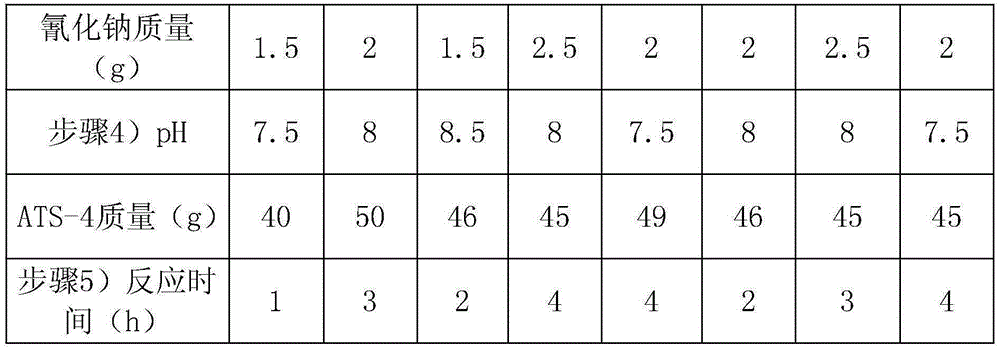
Embodiment 2-9
[0036] Each embodiment is basically the same as embodiment 1, the difference lies in table 1.
[0037] Table 1
[0038]
[0039]
[0040] Table 2 shows the immobilization rate of halohydrin dehalogenase and its ATS-5 production rate after immobilization in each embodiment.
[0041] Table 2
[0042]
[0043] Note: where "the first time" is the generation rate of directly using the halohydrin dehalogenase immobilized in sodium alginate to catalyze the synthesis of (R)-4-cyano-3-hydroxybutyric acid ethyl ester, "the second "Times" is the generation rate of sodium alginate immobilized halohydrin dehalogenase used to catalyze the synthesis of ethyl (R)-4-cyano-3-hydroxybutyrate after the completion of the first synthesis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com