Streptococcus pneumoniae quantum dot immunochromatographic assay detection card and preparing method and application thereof
A streptococcus pneumoniae and immunochromatography technology, applied in the field of medical testing, can solve problems such as complicated operation steps, high quality requirements for sample materials to be tested, false positives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
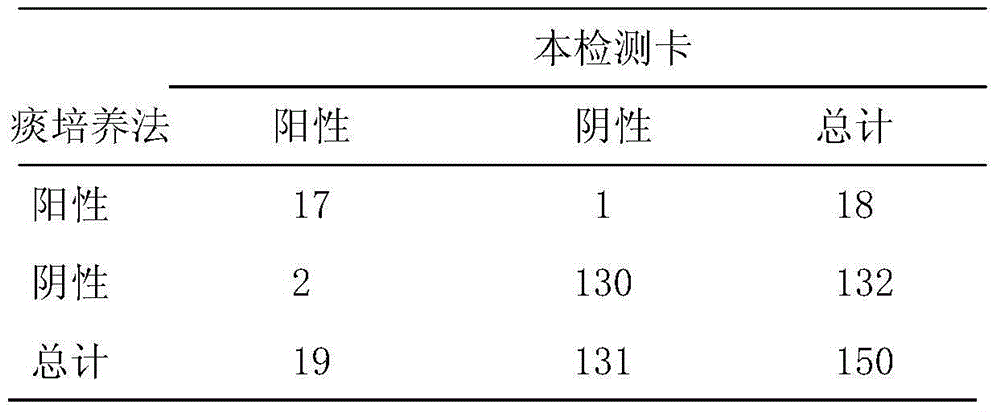
Examples
preparation example Construction
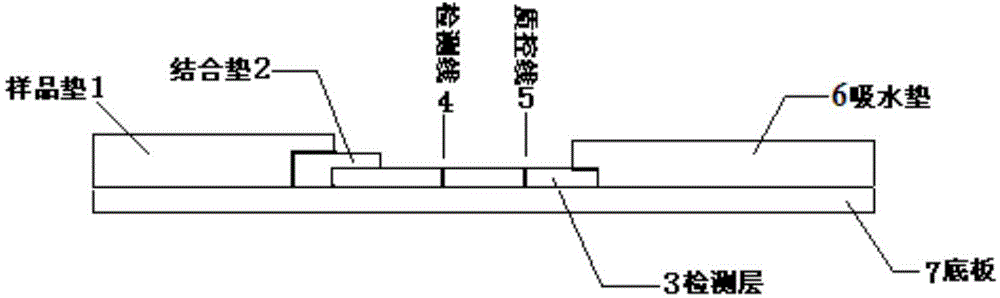
[0082] 1. Preparation of conjugated pads
[0083] (1) Preparation and purification of recombinant PspA1-His and PspA2-His fusion proteins:
[0084] Bioinformatics analysis was performed on the Fam1PspA and Fam2PspA proteins of human Streptococcus pneumoniae, and the peptides with the most abundant antigenic epitopes in the extracellular domains of the Fam1PspA and Fam2PspA proteins of Human Streptococcus pneumoniae were respectively obtained, and their corresponding gene sequences were found; The 5' end and 3' end of the sequence were respectively introduced with enzyme cutting sites, and the whole gene sequence was chemically synthesized respectively, and marked as PspA1 and PspA2 at the same time. See the sequence listing for their gene sequences. The two gene sequences were respectively cloned into the expression vector pET-28a(+) according to conventional methods, and then recombinant PspA1-His and PspA2-His fusion proteins were expressed. These two fusion proteins both ...
Embodiment 1
[0128] Embodiment 1 (preparation embodiment)
[0129] Conjugate pad preparation
[0130] (1) Preparation and purification of recombinant PspA1-His and PspA2-His fusion proteins
[0131] 1. Cloning of related genes
[0132] Bioinformatic analysis was performed on the Fam1PspA and Fam2PspA proteins of human Streptococcus pneumoniae (the accession numbers in the NCBI protein database are AAF27703 and AAF27712), respectively, to obtain the peptides with the most abundant antigenic epitopes in their extracellular domains, and to find their corresponding At the same time, the whole gene sequence was chemically synthesized after introducing the restriction site NdeI at the 5' end, the termination signal TAA and the restriction site XhoI at the 3' end (the whole sequence synthesis was completed by GenScript Biotechnology Co., Ltd. , the artificially synthesized gene fragments were connected to the vector pUC57 at the time of delivery), which were denoted as PspA1 and PspA2. The ful...
Embodiment 2
[0159] Embodiment 2 (preparation embodiment)
[0160] Preparation of sample pads
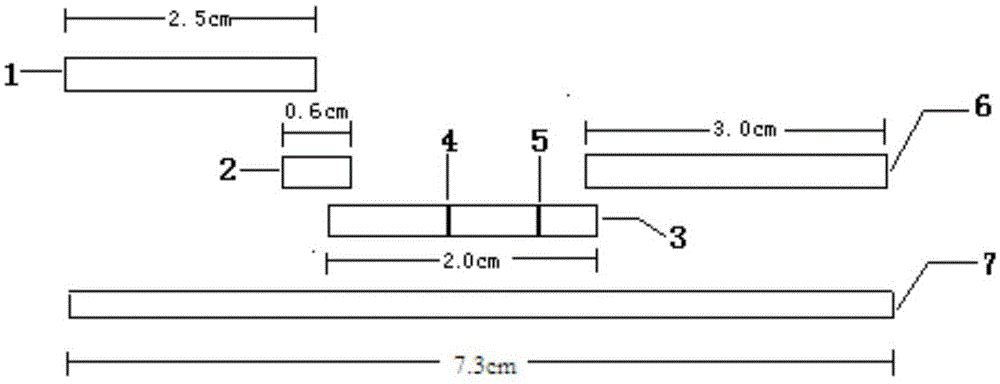
[0161] Prepare sample pad treatment solutions with different formulations, observe the release effect of quantum dot-labeled antibodies, and optimize through multiple orthogonal experiments to obtain the optimal sample pad treatment solution formulation (that is, described in the present invention). Take a piece of glass cellulose membrane, soak it in the sample pad treatment solution for at least 3 hours, then place it in a biological safety cabinet at 37°C, ventilate and dry it, and cut it into a size of 4cm*2.5cm / strip to prepare the sample pad , Store in airtight and dry place at 25°C. Tests have proved that the use of the sample pad greatly improves the release rate of the quantum dot-labeled antibody on the binding pad and achieves a better application effect.
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com