Method for preparing nanometer barium sulfate by using molecule mixing strengthening reactor
A nano-barium sulfate and reactor technology, which is applied in the direction of nanotechnology, nanotechnology, nanotechnology, etc. for materials and surface science, can solve the problems of high difficulty in purification, large particle size of products, and complex post-processing, etc., to achieve improved Nucleation rate, inhibition of particle size growth, and effect of increasing gloss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
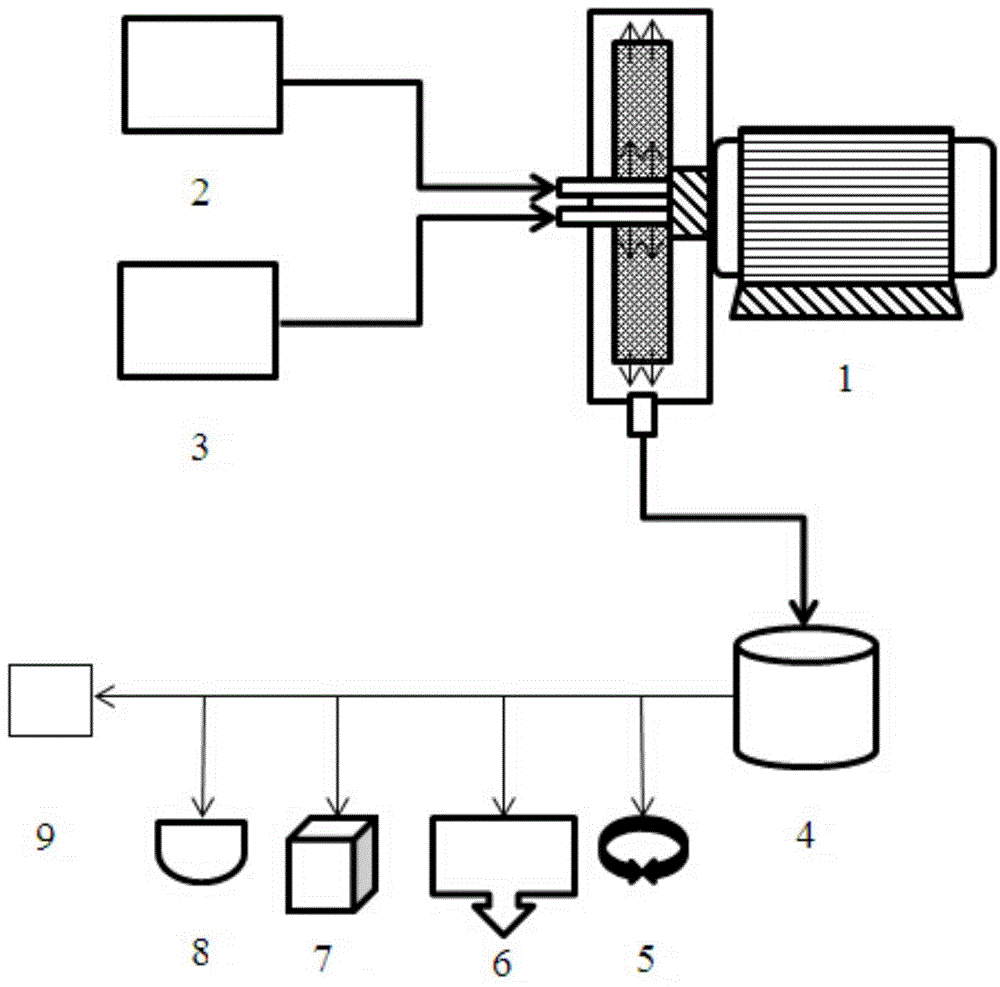
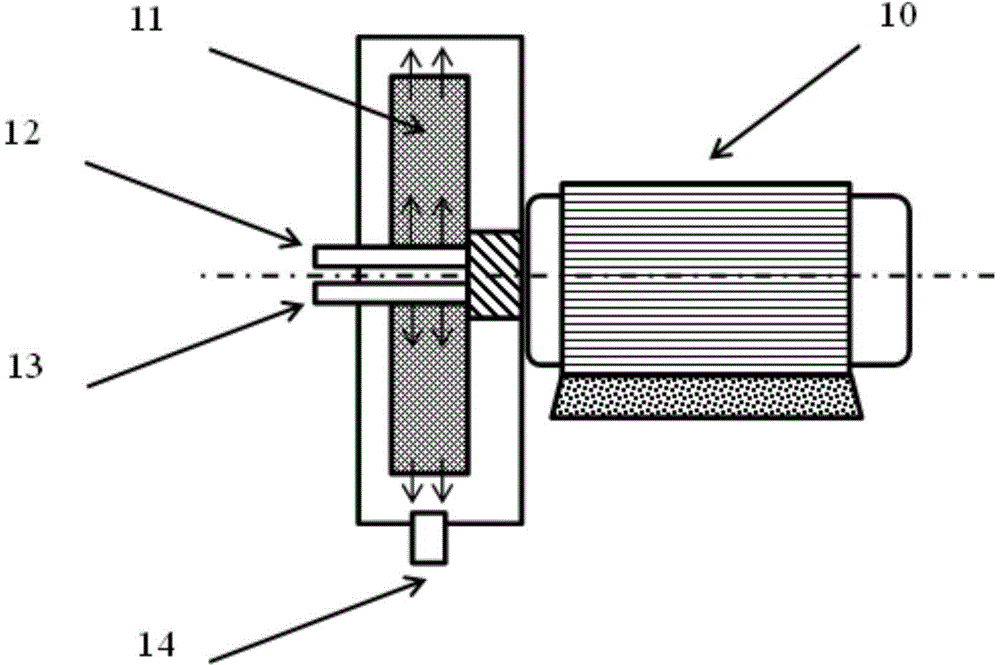
[0047] 1, the high gravity rotating packed bed reactor used in the present invention is prior art, such as disclosed patent (ZL952154307); The reaction scheme of a kind of embodiment of the high gravity rotating packed bed reactor that the present invention adopts is as figure 1 As shown, the schematic diagram of the high-gravity rotating packed bed reactor is shown in figure 2 shown. The implementation is as follows:
[0048] Turn on the supergravity rotating device 1, and adjust the rotating speed so that the rotating speed in the rotor of the supergravity device reaches a predetermined value. The barium salt solution in the barium salt solution storage tank 2 is pumped into the barium salt solution feed port 12 in the supergravity rotary device through a pump; the sulfate solution in the sulfate solution storage tank 3 is pumped into the high gravity rotary device through a pump Sulfate solution feed port 13; the rotor 11 in the supergravity device is driven by the motor...
Embodiment 1
[0053] The barium chloride molar concentration is 1.5mol / L aqueous solution 4000ml joins in the barium salt solution storage tank; The sodium sulfate molar concentration is 1.5mol / L aqueous solution 4000ml joins in the sulfate solution storage tank;
[0054] Turn on the high-gravity rotating device, adjust the speed to 1000rpm; turn on the feed pump, and simultaneously transport the barium salt solution and the sulfate solution to the rotating bed for precipitation and crystallization reaction, and control the feeding rate of the barium salt solution and the sulfate solution. The temperature of the reaction system is 1000ml / min, and the temperature of the reaction system is controlled to be 25°C; after the feeding of the barium salt solution and the sulfate solution is completed, and the suspension obtained from the reaction flows out of the supergravity rotating device, close the supergravity rotating device;
[0055] Centrifuge the obtained suspension, adjust the rotation spe...
Embodiment 2
[0059] The barium chloride molar concentration is that 5000ml of the aqueous solution of 1.2mol / L is added in the barium salt solution storage tank; The aqueous solution 5000ml that the ammonium sulfate molar concentration is 1.2mol / L is joined in the sulfate solution storage tank;
[0060]Turn on the high-gravity rotating device, adjust the speed to 1200rpm; turn on the feed pump, and simultaneously transport the barium salt solution and the sulfate solution to the rotating bed for precipitation and crystallization reaction, and control the feeding rate of the barium salt solution and the sulfate solution. The temperature of the reaction system is 800ml / min, and the temperature of the reaction system is controlled to be 30°C; after the feeding of the barium salt solution and the sulfate solution is completed, and the suspension obtained from the reaction flows out of the supergravity rotating device, close the supergravity rotating device;
[0061] Centrifuge the obtained susp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com