Monoclonal antibody against phosphomannose isomerase (PMI) protein and application thereof
A monoclonal antibody and protein technology, applied in anti-enzyme immunoglobulin, measuring devices, instruments, etc., to achieve extremely strong specificity and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
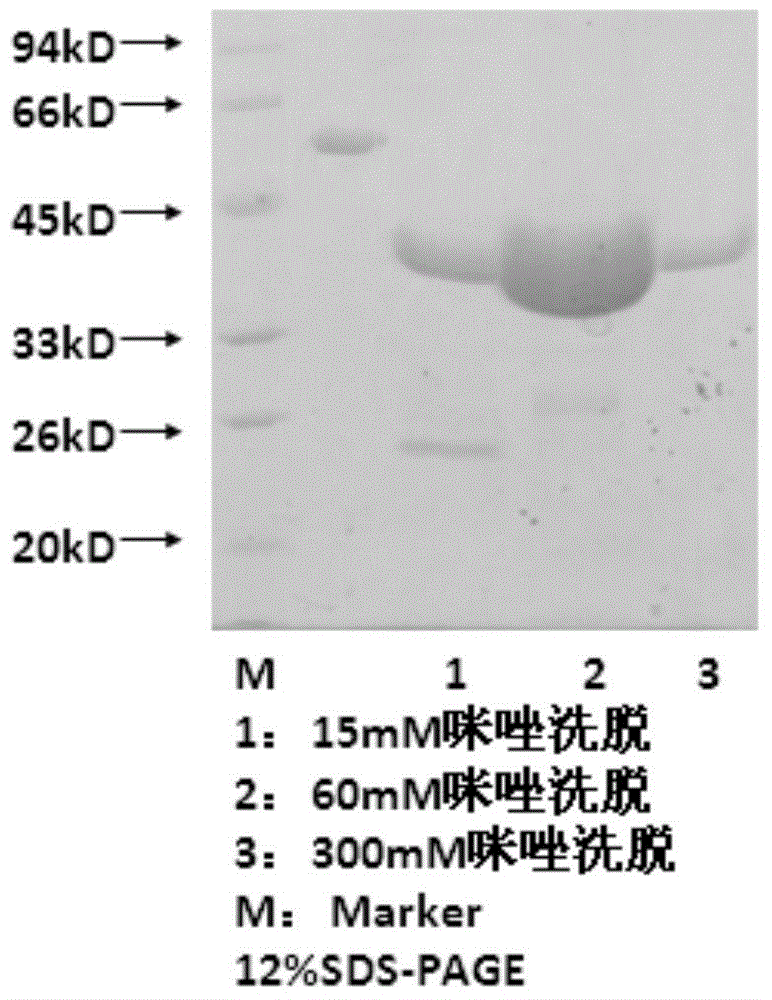
[0028] The preparation of embodiment 1 recombinant PMI protein
[0030] The nucleic acid sequence of NC_004088.1 in PMI monoclonal Genbank, design specific upstream primer PMI-F: TATGTACATATGCAAAAACTCATTAACTC specific downstream primer PMI-R: AGTGCTCGAGCTTGTTGTAAACACGC, amplified from the reverse transcription product in lymphocytes, including coding from position 411 to The gene fragment of the 750th amino acid. During the PCR process, NdeI and XhoI restriction sites were added to the 5' and 3' ends of the gene, respectively. The PCR product was separated by agarose gel electrophoresis and recovered. The recovered fusion protein gene and the plasmid vector pET-30a used for expression were digested with NdeI and XhoI respectively, recovered by electrophoresis again, and ligated with T4DNA ligase. The ligation product was transformed into Escherichia coli competent cell BL21, the clones on the plate were picked and inoculated, the plasmid DNA was extrac...
Embodiment 2
[0033] The establishment of embodiment 2 hybridoma cell lines
[0034] 1. Immunity
[0035] The cross-linked polypeptide in Example 1 was emulsified with complete Freund's adjuvant (Sigma Company), and immunized with 4-6 week-old female Balb / c mice (purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.), subcutaneously in the abdomen. Inject each mouse at 6 points with a dose of 60 μg / mouse. Immunization was boosted every 14 days, and the antigen was emulsified with Freund's incomplete adjuvant (Sigma Company) at a dose of 30 μg per mouse. 7 days after the third booster immunization, indirect ELISA (wavelength 450nm) was used to detect the polyantibody titer of the anti-immunogen in the mouse serum. 50μg / only.
[0036] 2. Cell Fusion
[0037] Aseptically prepare the mouse splenocyte suspension that reaches the immune standard, mix it with mouse myeloma cell sp2 / 0 (ATCC) at a ratio of 5:1, and centrifuge at 1500rpm for 5min. After the supernatant wa...
Embodiment 3
[0042] Example 3 Preparation of Monoclonal Antibody by Ascites Induction Method
[0043] 1. Ascites preparation
[0044] Cells in the logarithmic growth phase were washed with serum-free medium and suspended, counted to 5×10 5 , 1ml. The suspended cells were injected intraperitoneally into mice previously sensitized with paraffin oil. Ascites collection was started 7 days later. The removed ascites was centrifuged at 4000rpm for 10min at 4°C. Carefully suck out the ascites in the middle and collect in a centrifuge tube, and store at 4°C or -20°C.
[0045] 2. Purification of monoclonal antibodies
[0046] Antibody was purified from ascitic fluid by HiTraprProteinAFF (GE Company) affinity chromatography according to the instructions. The purity was identified by SDS-PAGE gel, and the concentration was determined by Bradford method. Purified antibodies were stored at -20°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com