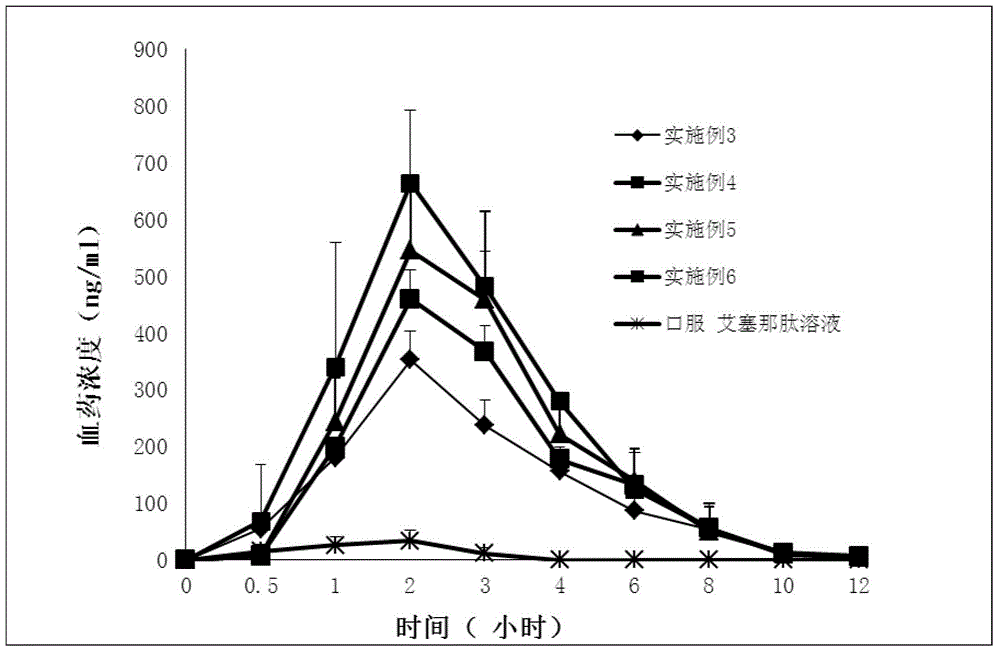
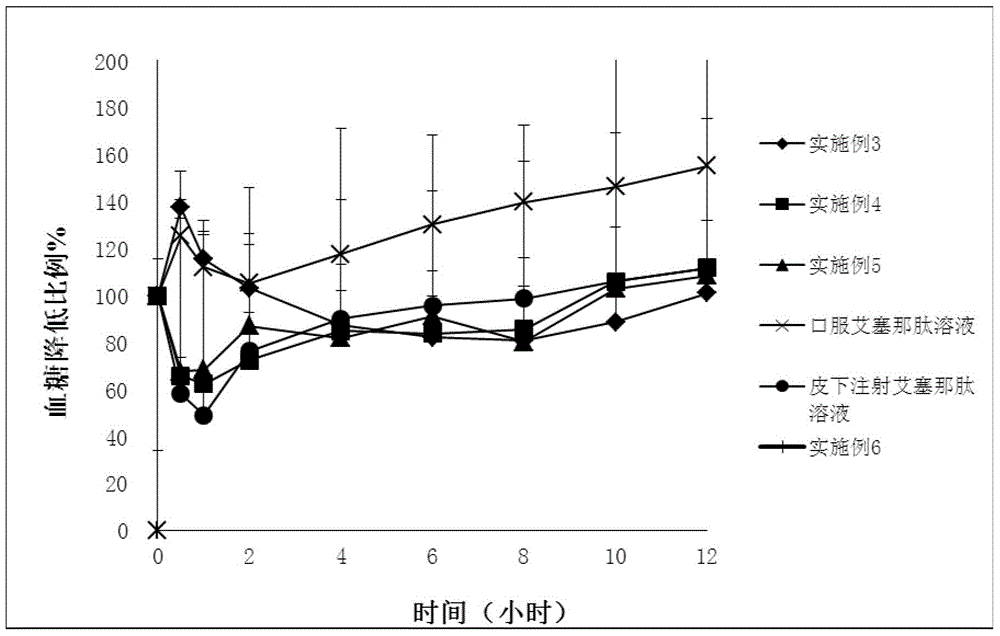
Exenatide oral nanoparticle
A technology of exenatide and nanoparticles, which is applied in the field of exenatide-containing oral nanoparticles and its preparation, which can solve the problems of poor fat solubility, chemical and conformational instability, and large molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] The synthesis of embodiment 1CSK polypeptide
[0016] 1. Resin swelling
[0017] Put 100% resin into the reaction tube, add 1500ml dimethylformamide (DMF), and swell for 30min.
[0018] 2. Deprotection
[0019] Remove DMF, add 1500ml 20% piperidine DMF solution, remove after 5min, add 1500ml 20% piperidine DMF solution, 15min.
[0020] 3. Detection
[0021] Take out the piperidine solution, take more than a dozen resins, wash with ethanol three times, add ninhydrin, potassium cyanide (KCN), and one drop of phenol solution, and heat at 105C-110C for 5min.
[0022] 4. Washing
[0023] Wash twice with 1000ml DMF, methanol and DMF respectively.
[0024] 5. Condensation
[0025] According to the amino acid sequence of the required polypeptide, add 10mmol amino-terminal protected amino acid, 30mmol coupling agent 2-(7-azobenzotriazole)-tetramethyluronium hexafluorophosphate (HBTU), use as little DMF as possible Dissolve, add to the reaction tube, and immediately add mo...
Embodiment 2
[0034] The preparation of embodiment 2CSK-chitosan
[0035] 1. Dissolve 100mg of the purified peptide in PBS, soak in ice bath for 15min and set aside.
[0036] 2. Add 200 mg of 1-ethyl-3-(dimethylaminopropyl) carbodiimide hydrochloride (EDC) and 200 mg of N-hydroxysuccinimide (NHS) to the polypeptide PBS solution in 1. ) Low temperature reaction for 1h.
[0037] 3. Add 100 mg of chitosan to the reaction solution, and react for 4 hours.
[0038] 4. Prepare 2000ml of phosphate buffer solution with a pH of 7.4.
[0039] 5. Pack the reaction solution in a dialysis bag and tie it tightly, and dialyze it in the buffer solution for 48 hours.
[0040] 6. Freeze-dry the dialyzed product to obtain CSK-chitosan (1:1).
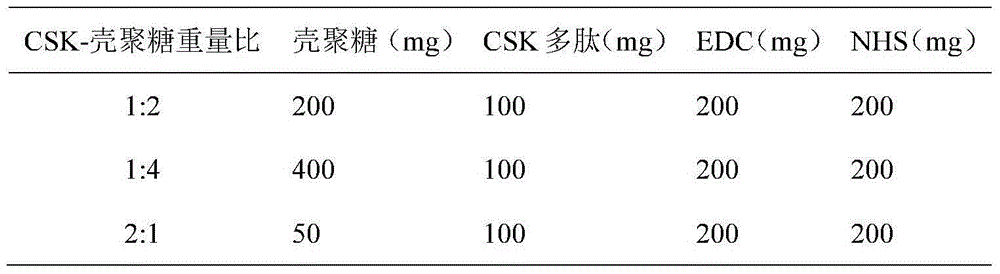
[0041] Other weight ratios of CSK-chitosan adopt the same method as above, and the required reagents and consumption are shown in Table 1:
[0042] Table 1 prepares the required reagents and consumption of different weight ratios of CSK-chitosan
[0043]
Embodiment 3
[0045] 10ml1mg / ml chitosan solution (dissolved in 0.25% acetic acid), under magnetic stirring, add 0.5ml2mg / mlExenatide solution under gravity with 5ml syringe, add 2.5mg / mlTPP aqueous solution 1.8ml under gravity with 5ml syringe, Stir with a magnetic stirrer at 500 rpm for 50 min, and centrifuge at 3500 rpm for 30 min. Add 0.1 mg of preservative potassium sorbate and 1 mg of sodium malate flavoring agent to the prepared nanoparticle solution to obtain the nanoparticle oral liquid preparation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com