Chinese herb pills used for treating malabsorption syndrome and preparation method thereof
A traditional Chinese medicine pill and malabsorption technology, which can be used in pharmaceutical formulations, medical raw materials derived from birds, medical preparations containing active ingredients, etc. therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] The raw material composition and the weight of active ingredient of medicine of the present invention are:
[0069] 66-82 parts of wild water chestnut 63-79 parts of radish seed 60-76 parts of gorgon fruit 57-72 parts of chicken dung vine
[0070] 53-69 servings of jujube, 50-66 servings of longan meat, 46-63 servings of Guan Zhong, 43-60 servings of Chiyangzi
[0071] 40-57 parts of hawthorn, 38-54 parts of psoralen, 35-51 parts of Guya, 33-48 parts of gallinacea
[0072] 30-45 servings of sleeping vegetables, 27-42 servings of white stone fat, 25-39 servings of angelica, 22-36 servings of wild Magnolias
[0073] 19-32 parts of black medicine Schisandra 16-28 parts Alisma 13-25 parts Red cardamom 10-22 parts
[0074] 8 to 18 parts of tangerine peel, 5 to 15 parts of atractylodes rhizome, 3 to 12 parts of licorice, 1 to 10 parts of myrobalan.
[0075] The processing steps of the pharmaceutical preparation method of the present invention are:
[0076] (1) Wash and dr...
Embodiment 2
[0088] The raw material composition and the weight of active ingredient of medicine of the present invention are:
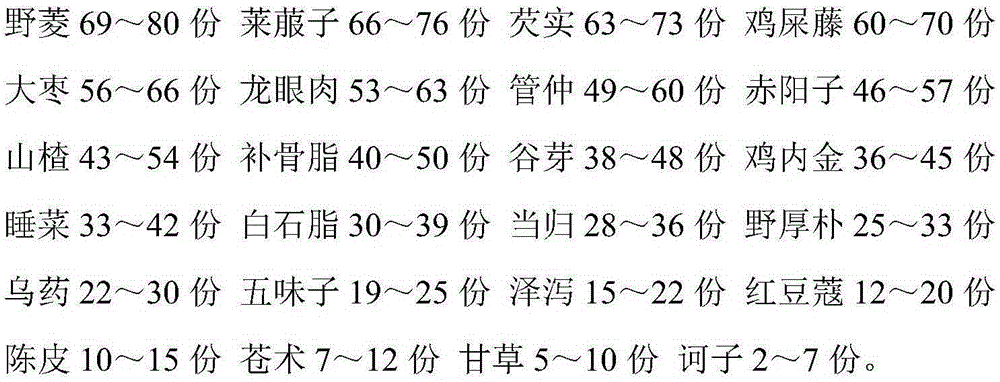
[0089] 69-80 parts of wild water chestnut 66-76 parts of radish seed 63-73 parts of gorgon 60-70 parts of chicken dung vine
[0090] 56-66 servings of jujube, 53-63 servings of longan meat, 49-60 servings of Guan Zhong, 46-57 servings of Chiyangzi
[0091] 43-54 parts of Hawthorn, 40-50 parts of Psoralen, 38-48 parts of Gu Ya, 36-45 parts of Chicken Gold
[0092] 33-42 servings of sleeping vegetables, 30-39 servings of white stone fat, 28-36 servings of angelica, 25-33 servings of wild officinalis
[0093] 22-30 parts of black medicine Schisandra 19-25 parts Alisma 15-22 parts Red cardamom 12-20 parts
[0094] 10-15 parts of tangerine peel, 7-12 parts of atractylodes rhizome, 5-10 parts of licorice, 2-7 parts of myrobalan.
[0095] The process steps, usage and dosage of the medicine preparation method in this embodiment are the same as those in Example 1.
Embodiment 3
[0097] The raw material composition and the weight of active ingredient of medicine of the present invention are:
[0098] 74 parts of wild water chestnut, 71 parts of radish seed, 68 parts of gorgon fruit, 65 parts of chicken dung vine
[0099] 62 parts of jujube, 58 parts of longan meat, 55 parts of Guan Zhong, 52 parts of Chiyangzi
[0100] 49 parts of hawthorn, 46 parts of psoralen, 43 parts of grain buds, 40 parts of chicken gold
[0101] 37 servings of sleeping vegetables, 34 servings of white stone fat, 32 servings of angelica, 29 servings of wild Magnolias
[0102] 26 parts of Wuyao 22 parts of Schizandra 19 parts of Alisma 16 parts of Red Cardamom
[0103] 12 parts of tangerine peel, 9 parts of atractylodes rhizome, 7 parts of licorice, and 4 parts of myrobalan.
[0104] The process steps, usage and dosage of the medicine preparation method in this embodiment are the same as those in Example 1.
[0105] clinical information:
[0106] 1. Case selection
[0107] 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com