Amphiphilic molecules with aggregation-induced emission effects, preparing method thereof and uses of the amphiphilic molecules
A technology of aggregation-induced luminescence and amphiphilic molecules, which can be used in luminescent materials, material excitation analysis, chemical instruments and methods, etc. The effect of stable luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
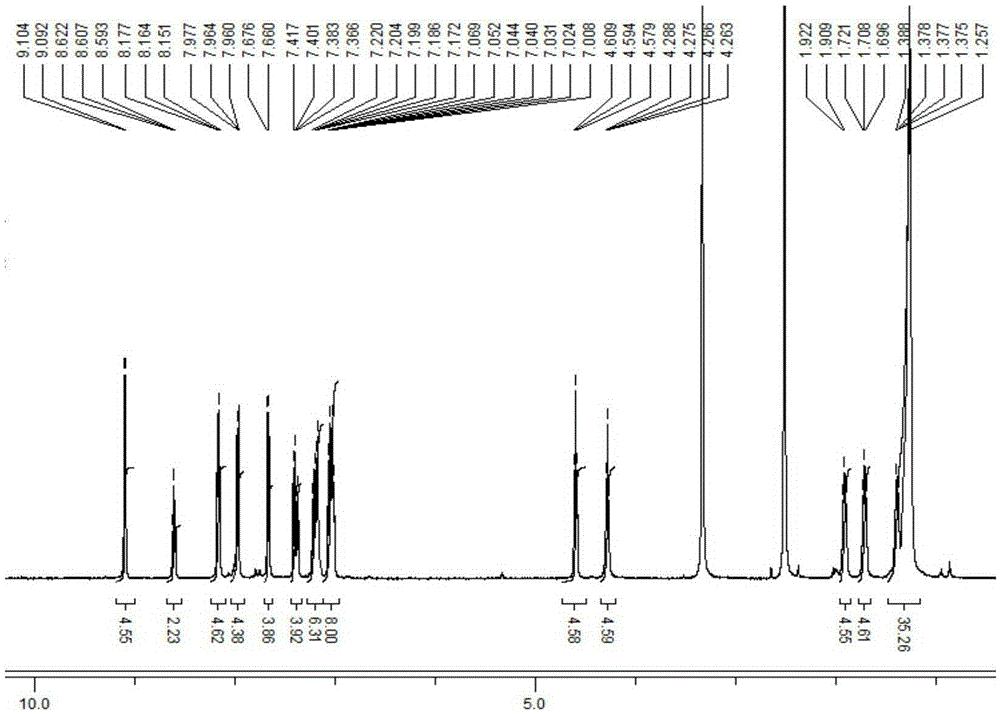
[0035] Example 1: Synthesis of TPE-11.
[0036] (1) Under the protection of argon, 400mg (0.82mmol) of 1-bromo-4-(2-(4-bromophenyl)-1,2-diphenylvinyl)benzene, 622mg (1.64mmol) 4 -11-bromoundecyl ethynylbenzoate, 100mg (0.08mmol) tetrakis(triphenylphosphine) palladium, 20mg (0.08mmol) cuprous iodide were added to a 100mL three-necked flask containing 50mL of tetrahydrofuran, Add 5mL (36.07mmol) of triethylamine under stirring conditions, heat to 70°C and reflux for 10 hours. After the reaction is completed, tetrahydrofuran is distilled off under reduced pressure. The residue is extracted with a water / dichloromethane system and the organic phase is separated. Dry over anhydrous sodium sulfate for more than 0.5 hours, distill under reduced pressure to remove dichloromethane and obtain a crude product. Purify by silica gel column chromatography with ethyl acetate: petroleum ether=1:10 (v / v) to obtain a yellow solid The yield is 8%;
[0037]
[0038] (2) Add the intermediate obtained ...
Embodiment 2
[0043] Example 2: Synthesis of TPE-11.
[0044] (1) Under the protection of nitrogen, mix 200mg (0.41mmol) of 1-bromo-4-(2-(4-bromophenyl)-1,2-diphenylvinyl)benzene, 311mg (0.82mmol) of 4- Ethynyl benzoic acid 11-bromoundecyl ester, 38mg (0.04mmol) bis(triphenylphosphine) palladium dichloride, 10mg (0.04mmol) cuprous iodide were added to a 100mL three-necked flask containing 40mL tetrahydrofuran Add 3mL (21.64mmol) of triethylamine under stirring conditions, heat to 85°C and reflux for 20 hours. After the reaction is completed, tetrahydrofuran is distilled off under reduced pressure. The residue is extracted with a water / dichloromethane system to separate the organic Phase, dried with anhydrous sodium sulfate for more than 0.5 hours, distilled under reduced pressure to remove dichloromethane to obtain a crude product, and purified by silica gel column chromatography using ethyl acetate: petroleum ether = 1:10 (v / v) eluent Yellow solid intermediate, the yield is 6%;
[0045]
[00...
Embodiment 3
[0047] Example 3: Determination of the fluorescence properties of TPE-11.
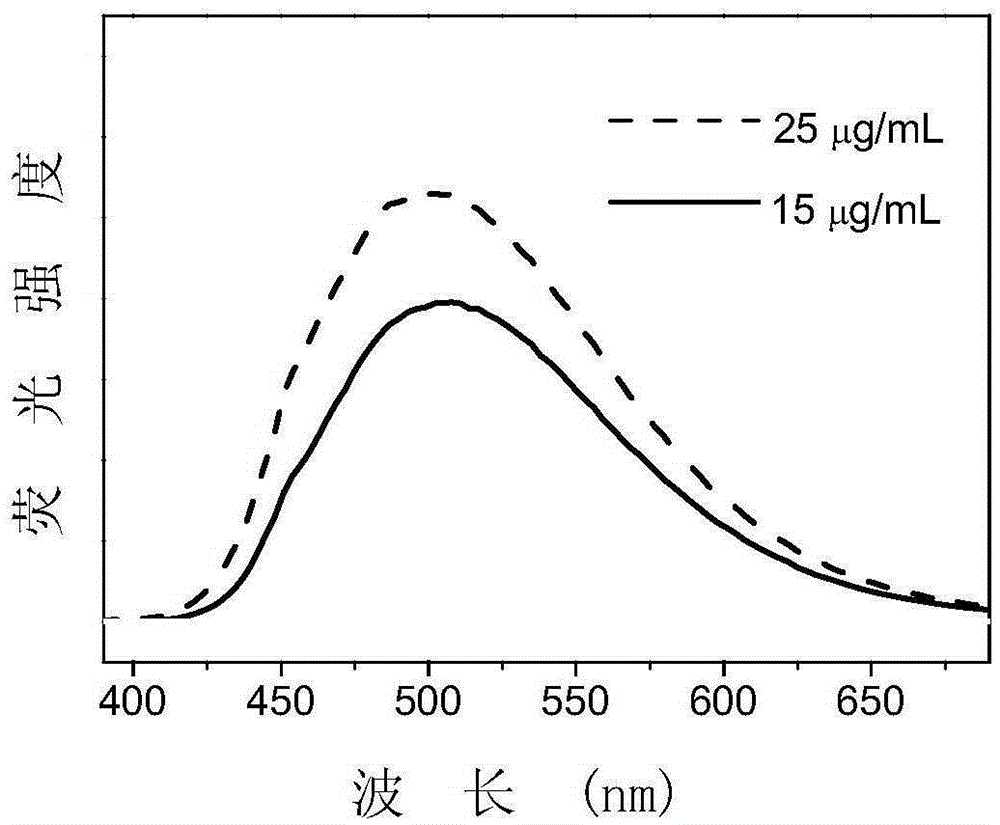
[0048] Dissolve 2mg TPE-11 in 2mL deionized water to prepare a TPE-11 aqueous solution mother liquor with a concentration of 1mg / mL, dilute to a concentration of 25 and 15μg / mL, and excite at 360nm wavelength to obtain the fluorescence emission spectrum of TPE-11. Such as figure 2 Shown.
[0049] From figure 2 It can be seen that the synthesized amphiphilic molecule TPE-11 has a strong fluorescence emission peak around 500nm, indicating that the molecule can emit green fluorescence in an aqueous solution. In addition, the fluorescence intensity of high concentration (25μg / mL) TPE-11 is higher than that of low concentration (15μg / mL) TPE-11, indicating that TPE-11 has a typical aggregation-induced luminescence effect.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com