Catalyst for polylactone preparation
A technology of catalyst and polylactone, which is applied in the field of catalyst for preparing polylactone, and can solve the problems of inapplicability and difficult removal of metal residues, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In a 1-liter glass bottle, add 300 grams of lactide and 2 grams of ethylene glycol, replace it with high-purity nitrogen three times, then add 0.05 grams of 1-(4-sulfonic acid) butyl-3-methyl under nitrogen protection Imidazole tetrafluoroborate, heated to 130-160 degrees and reacted for 12 hours, then cooled to room temperature, dissolved the product with 2000 ml of chloroform, precipitated with 5000 ml of ethanol to obtain 280 grams of polylactic acid, and the weight average molecular weight was 20,000 as determined by GPC. The molecular weight distribution 1.3.
Embodiment 2
[0023] In a 3-liter glass bottle, add 300 grams of lactide, replace it with high-purity nitrogen three times, add 1000 ml of tetrahydrofuran, 1.5 grams of 1-(3-sulfonic acid) propyl-3-methylimidazolium tetrafluoro Borate, heated to 80-100 degrees and reacted for 12 hours, then cooled to room temperature, precipitated with 5000 ml of ethanol to obtain 240 grams of polylactic acid, with a weight average molecular weight of 110,000 and a molecular weight distribution of 1.28 as determined by GPC.
Embodiment 3
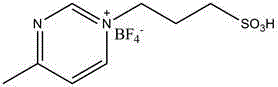
[0025] In a 3-liter glass bottle, add 300 grams of trimethylene carbonate, replace it with high-purity nitrogen three times, then add 1000 ml of toluene and 15 grams of 1-(4-sulfonic acid) butyl-3-methylpyridine under nitrogen protection. The pyrazine tetrafluoroborate was heated to 80-100 degrees and reacted for 12 hours, then cooled to room temperature, and precipitated with 5000 milliliters of ethanol to obtain 260 grams of polytrimethylene carbonate. The weight average molecular weight was 150,000 as determined by GPC, and the molecular weight distribution was 1.32.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com