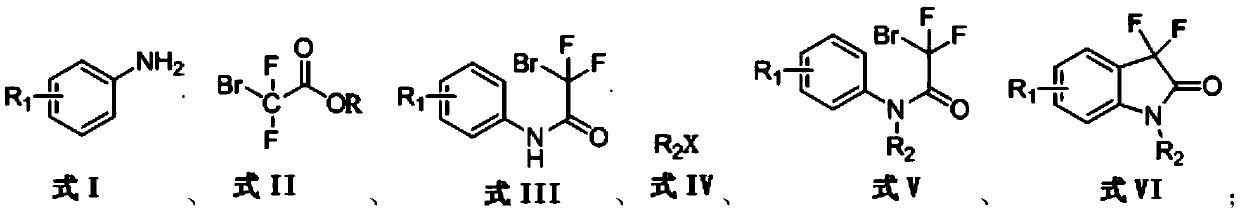
Synthetic method for 3,3-difluoro-2-oxindole derivative
A technology for indole derivatives and synthesis methods, which is applied in the field of synthesis of 3,3-difluoro-2-oxindole derivatives, can solve problems such as increased synthesis costs and drug research and development costs, and achieves reduction in usage, The effect of safe use and low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
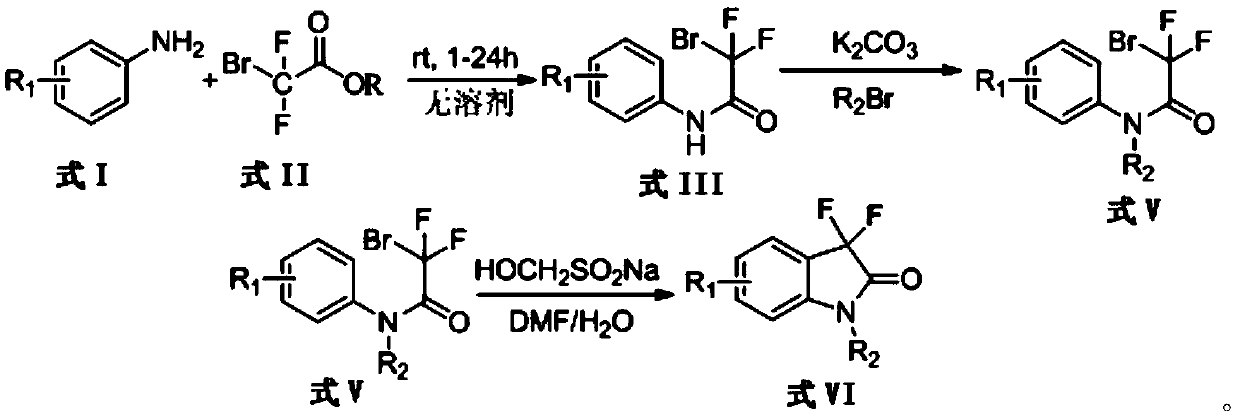
[0027] Target product I-1 is The synthesis steps are as follows:
[0028] 1. Synthesis of Aromatic Amides
[0029]Weigh 1.23g (10mmol) of p-methoxyaniline and add it to a 100mL single-necked round-bottomed flask, add 1.92mL (15mmol) of ethyl difluorobromoacetate respectively, put in a magnet, and under nitrogen protection, at 25°C Stir for 6 hours, stop stirring; add 30mL water and 50mL ethyl acetate to the flask, stir for another 5 minutes, transfer the solution to a separatory funnel, and the solution is separated after the reaction; The ester layer was washed once with dilute hydrochloric acid aqueous solution (0.5mol / L) and once with saturated brine; dried over anhydrous sodium sulfate, and evaporated the solvent under reduced pressure at 45°C to obtain light yellow powder 2-bromo-2,2-di Fluoro-N-(4-methoxyphenyl)acetamide 2.72 g (97% yield).
[0030]
[0031] 2. Synthesis of N-alkyl protected aromatic amides:
[0032] 1.4g (5mmol) of 2-bromo-2,2-difluoro-N-(4-meth...
Embodiment 2
[0038] Target product I-2 is The only difference between the synthesis steps and Example 1 is that p-methylaniline is used instead of p-methoxyaniline.
Embodiment 3
[0040] Target product I-3 is The only difference between the synthetic steps and Example 1 is that p-methoxyaniline is replaced by aniline.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com