Method for preparing cycloxylidin
A technology of epoxyline and glycidyl, which is applied in the field of pesticide preparation, can solve the problems of little industrial value, cumbersome treatment process, and limited production and application, and achieve high practical value, simple post-treatment process, and reduce solids The effect of waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
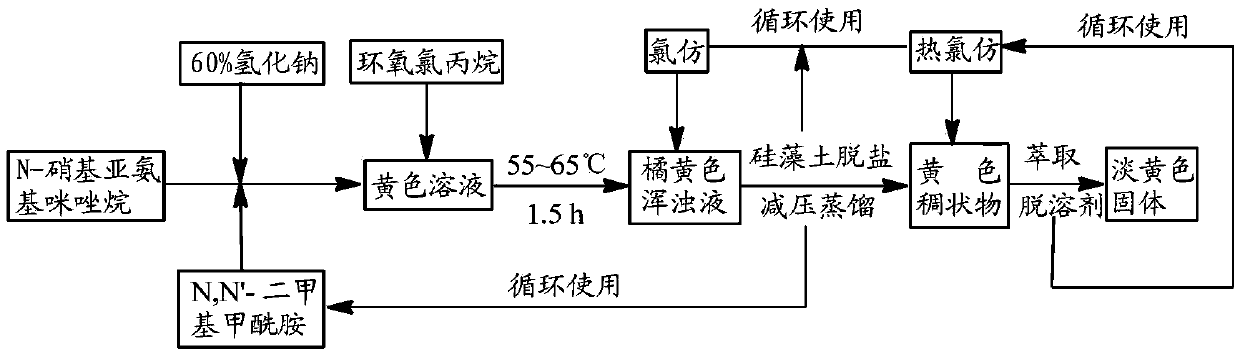
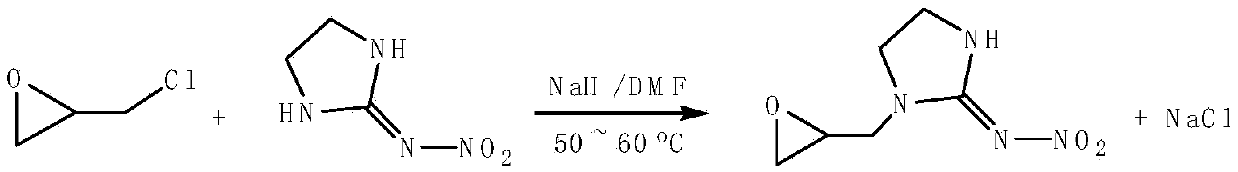
[0022] Embodiment 1: 97.5g (0.75mol) N-nitroimidazolidine, 18g (0.75mol) sodium hydride and 125mLN, N'-dimethylformamide and 75mL toluene solvent are added in the reaction flask, heated to 65°C, after the imidazolidin was basically dissolved, 46.3g (0.5mol) of epichlorohydrin was added dropwise, and the reaction was continued for 1.5h after the dropwise addition, and the reaction progress was tracked and monitored by TLC or HPLC until the end of the reaction. After the reaction is over, cool the reactant, add 100mL of chloroform, filter through diatomaceous earth to desalt, then distill the filtrate under reduced pressure to remove most of the solvent to obtain a thick solid, extract it with hot chloroform, collect the extract, remove Chloroform to give a light yellow solid product, dried to give a crude product, recrystallized from a hot ethanol solution, dried to give a light yellow 1-(2,3-epoxypropyl)-N-nitroimidazolidin-2-ylamine solid Product 77.3g, yield 83.2%, melting p...
Embodiment 2
[0023] Example 2: Add 97.5g (0.75mol) of N-nitroiminoimidazolidine, 18g (0.75mol) of sodium hydride and 175mL of N,N'-dimethylformamide solvent into the reaction flask, heat to 60°C, After the N-nitroimidazolidine was basically dissolved, 46.3 g (0.5 mol) of epichlorohydrin was added dropwise therein, and the reaction was continued for 2 h after the addition was completed, and the reaction progress was monitored by TLC or HPLC. After the reaction, the reactant was cooled and filtered, and the filtrate was distilled under reduced pressure to remove most of N,N'-dimethylformamide to obtain a viscous solid, which was washed with ether several times and filtered to obtain a light yellow solid , the crude product was recrystallized with hot acetone, and dried to obtain 78 g of light yellow 1-(2,3-epoxypropyl)-N-nitroimidazolidin-2-ylamine solid, with a yield of 83.9% and a melting point of: 87~90℃.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com