Test strip for detecting iprodione and preparation method and application of test strip
A technology of iprodione and test strips, which is applied in the field of test strips and preparations for detecting iprodione, can solve the problems of high detection cost and long detection time of analytical instrument methods, and achieve short detection time, simple storage, and specificity strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1, the composition of the test strip that detects iprodione
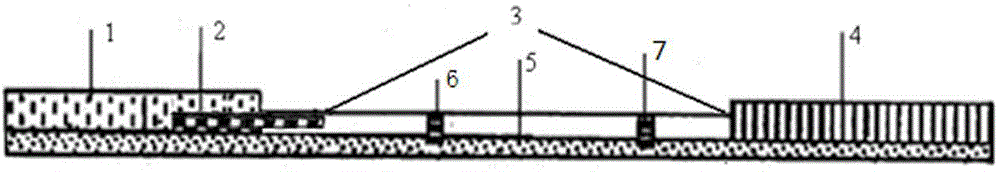
[0046] like figure 1As shown, the test strip for detecting iprodione according to the preferred embodiment of the present invention is composed of a sample absorbent pad 1 , a conjugate release pad 2 , a reaction film 3 , and a water absorbent pad 4 pasted on the bottom plate 5 in sequence. The end of the sample absorption pad is connected to the reaction membrane, the end of the reaction membrane is connected to the water absorption pad, the beginning of the sample absorption pad is aligned with the beginning of the bottom plate, and the end of the water absorption pad is aligned with the end of the bottom plate; there are detection areas 6 and Quality control area 7, the detection area and the quality control area are both strip-shaped vertical to the length of the test strip. The detection area is located on the side close to the sample absorbent pad, and the quality control area is located on ...
Embodiment 2
[0047] The preparation method of test strip described in embodiment 2, embodiment 1
[0048] 1. Preparation of Iprodione Hapten
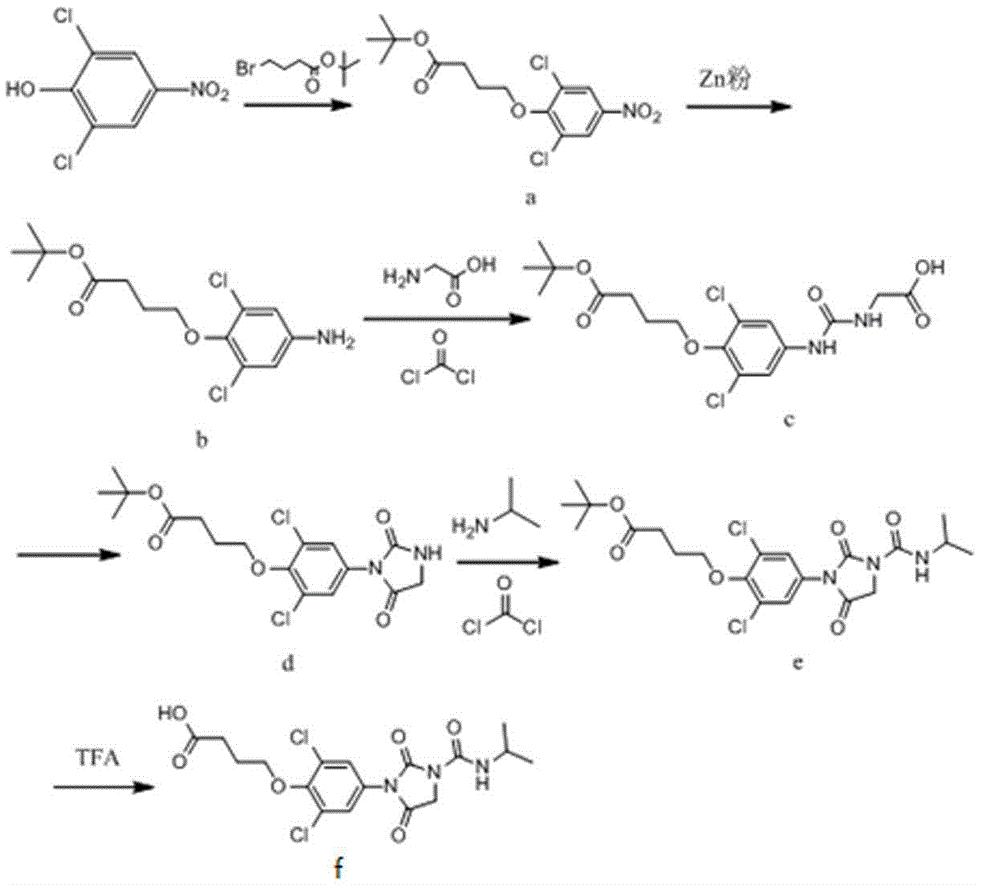
[0049] Synthesis of Compound A: Take 5.0g of 2,6-dichloro-4-nitrophenol and 3.2g of tert-butyl 4-bromobutyrate and stir in acetone, then add 2.6g of anhydrous potassium carbonate as a catalyst and react at 60°C 8h, after the reaction stopped, the solvent was evaporated to dryness, then extracted with water and ethyl acetate, dried with anhydrous sodium sulfate, concentrated, passed through a 200-300 mesh silica gel column, separated and purified by chromatography to obtain 5.23g of compound A, with a yield of 87% . 1HNMR (CDCl3, 300MHZ) δ: 7.945(1H,d,J=0.000),4.144(2H,t,J=7.500),7.945(1H,d,J=0.000),1.973(2H,tt,J=7.500 ,J=7.367),2.359(2H,t,J=7.367),1.414(3H),1.414(3H),1.414(3H,s).
[0050] Synthesis of Compound B: Add 3.1g of zinc powder and 1mL of glacial acetic acid to 20mL of water, react at 90°C for 30min, then add 5.2g of compound A in ethano...
Embodiment 3
[0099] Embodiment 3, the detection of iprodione residue in the sample
[0100] 1. Sample pretreatment
[0101] Tobacco leaf samples need to be warmed to room temperature 20-25°C before testing; weigh 1.0±0.05g of crushed samples into a polystyrene centrifuge tube; add 10mL of sample extract (50% methanol), and use a homogenizer to fully beat After standing still, pipette 0.1mL supernatant and 0.4mL sample diluent and mix to obtain the sample solution to be tested.
[0102] 2. Test with test strips
[0103] Use a pipette to take 100 μL of the sample solution to be tested (2-3 drops from the dropper) and drop it vertically into the sample hole. When the liquid flows, start timing, react for 10 minutes, and judge the result. If it exceeds 10 minutes, the result can be used as a reference.
[0104] 3. Analyze test results
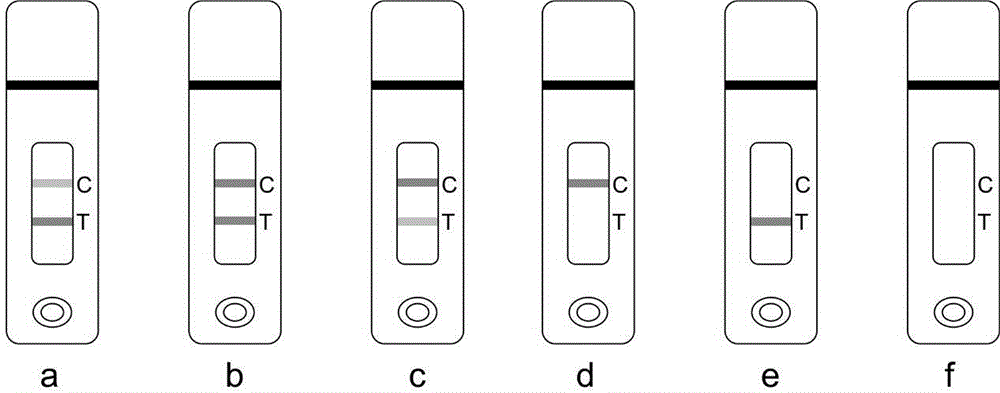
[0105] Negative (-): The color of the T line is darker than that of the C line or the color is consistent, which means that the concentration of iprodione i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com