Skin whitening composition containing arbutin derivative
A technology of arbutin and derivatives, applied in skin care preparations, cosmetic preparations, cosmetics, etc., can solve the problem of not finding p-hydroxyphenyl2
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
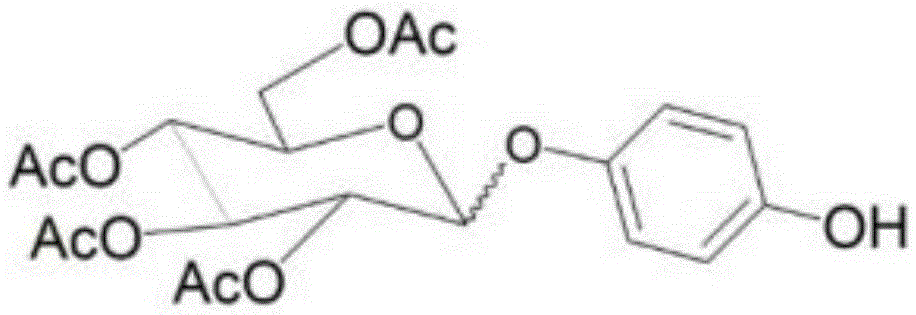
[0082] Example 1: Synthesis of arbutin derivative (p-hydroxyphenyl2,3,4,6-tetra-O-acethyl-β-D-glucoside)
[0083] Pentaacetylglucopyranoside (PAG) 390g (Sigma-Aldrich, USA), hydroquinone 110g (Sigma-Aldrich, USA), toluene 1L (Sigma-Aldrich, USA), triethylamine 10g (Sigma-Aldrich, USA) in a mixed solution Slowly add 0.1 g of the catalyst-p-toluenesulfonic acid hydrate (Sigma-Aldrich, USA), and make it react at a reaction temperature of 60°C for 5 hours while stirring vigorously. After the reaction, 2L of water and 3L of ethyl acetate were added, and the organic layer was separated and washed with water. The organic layer was removed from the solvent in a vacuum evaporator, and then separated according to silica gel column chromatography (columnchromatography), and after purification, 240 g of arbutin derivatives were obtained.
experiment example 1
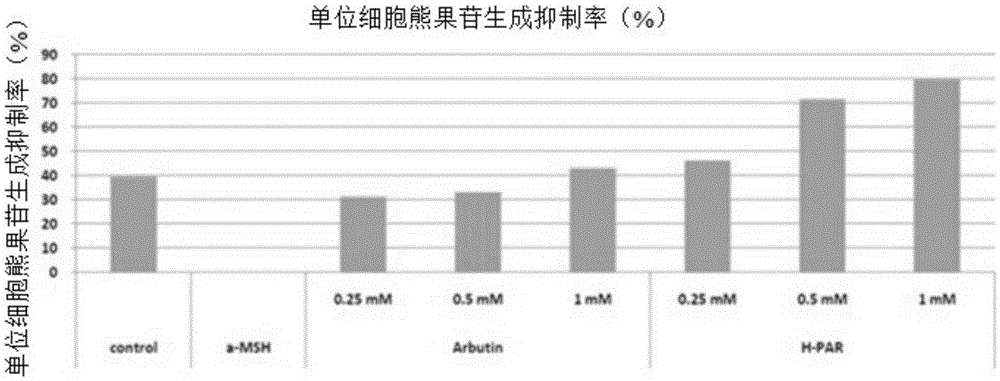
[0084] Experimental example 1: Mushroom tyrosinase activity hindering effect
[0085] The obstructive activity of mushroom tyrosinase is generally measured by spectroscopy, which is measured by our laboratory according to Vanni et al. (Vanni A. atal., Annalidi Chimica, 80(35), 1990). 0.1M potassium phosphate buffer (PotassiumPhosphatebuffer, pH6.8) 1.0ml, 0.3㎎ / ml L-tyrosine (L-tyrosine) aqueous solution 1.0ml and 1250unit / ml mushroom tyrosinase (mushroomtyrosinase) 0.1ml after mixing, in According to the concentration, 0.2ml of the sample solution was added, and the enzymatic reaction was carried out at 37°C for 10 minutes. The positive control group was arbutin (arbutin, Bioland). The absorbance of the reaction solution was measured at 480 nm, and the enzymatic interference activity of the sample was calculated according to the following formula 1, and the results are shown in Table 1.
[0086] [Formula 1]
[0087] Mushroom tyrosinase inhibition rate (%)=[(A-B) / A]x100
[0088] A: A...
experiment example 2
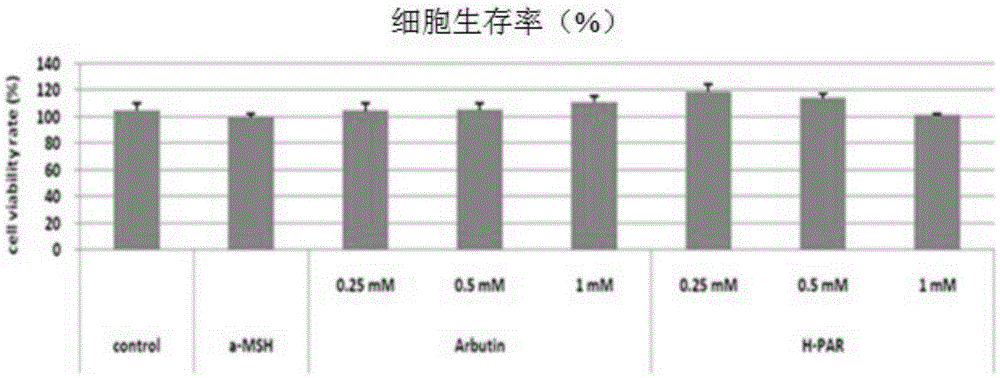
[0093] Experimental Example 2: Cytotoxicity assay using B16 melanoma cells
[0094] Using arbutin derivatives as samples, the cell viability of B16 melanoma cells was measured. The specific content is: B16 melanoma cells (ATCCCRL6323) in DMEM (Dulbecco'smodifiedEagle's Medium) medium supplemented with 10% small serum, with 1x10 5 Cell density is seeded, then in 5% CO 2 , Cultivate one day at 37℃. Then, after changing to a new DMEM10% medium, the samples were processed in wells of different concentrations and cultured for 3 days. After 3, remove the medium, treat 1ml of 0.33㎎ / ml MTT ((3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazoliumbromide), SigmaM5655), react at 37℃ for 4 hours , Then remove MTT, add 1mL of DMSO (dimethylsulfoxide), measure the degree of color development at 575nm as absorbance. All experiments were repeated 3 times, and the average was calculated after statistical processing. The results are shown in Table 2 and figure 1 Shown.
[0095]
[0096] [Table 2]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com