Sulfur modified porous iron oxide catalyst, preparation method therefor and application of sulfur modified porous iron oxide catalyst
An iron oxide and catalyst technology, applied in the field of environmental pollution remediation and inorganic materials, can solve the problems of loss of catalytic effect, narrow pH operating range, increased operating cost, etc., and achieve the effects of high reaction rate, large specific surface area and strong dispersibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
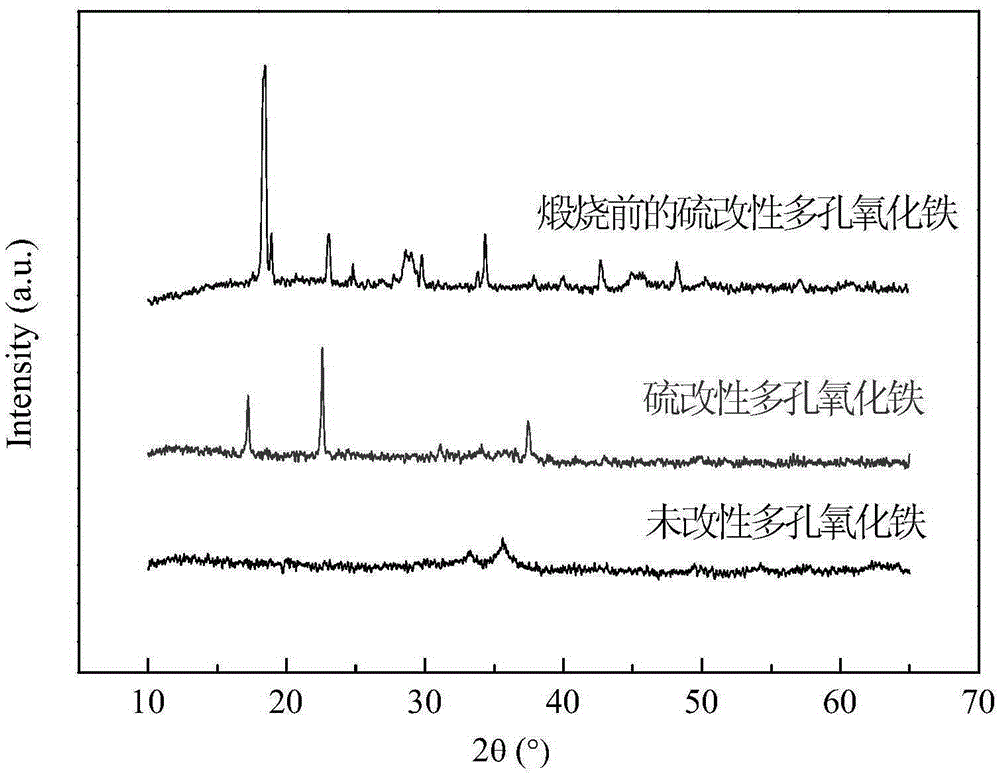
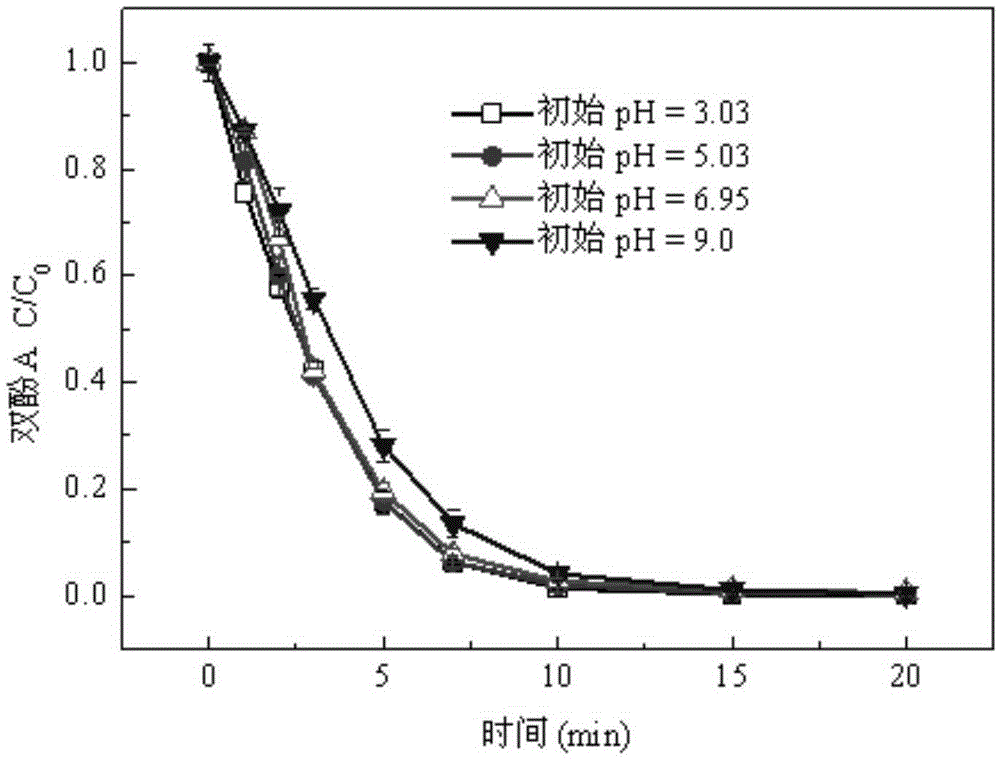
[0036] A sulfur-modified porous iron oxide catalyst is prepared by the following method: (1) 1.8 g of oxalic acid is dissolved in 50 mL of water to prepare an oxalic acid solution with a concentration of 0.4 mol / L, and the heating process is heated to 60° C. in a water bath. (2) Under magnetic stirring, add 0.01 mol of sodium thiosulfate to the above-mentioned oxalic acid solution, stir for 5 min and mix evenly, dropwise add 50 mL of a solution containing 0.02 mol of ferrous sulfate, and continue to stir the reaction Immediately after 30min, ice bath for 30min to obtain a yellow suspension; (3) filter the above-mentioned yellow suspension, and then dry it in a blast drying oven at 80° C. until the moisture is dried to obtain a yellow sulfur-modified oxyoxalate Iron precursor; the above precursor is calcined in a muffle furnace, and heated to 300°C at a heating rate of 4°C / min for 60min to obtain a sulfur-modified porous iron oxide catalyst.
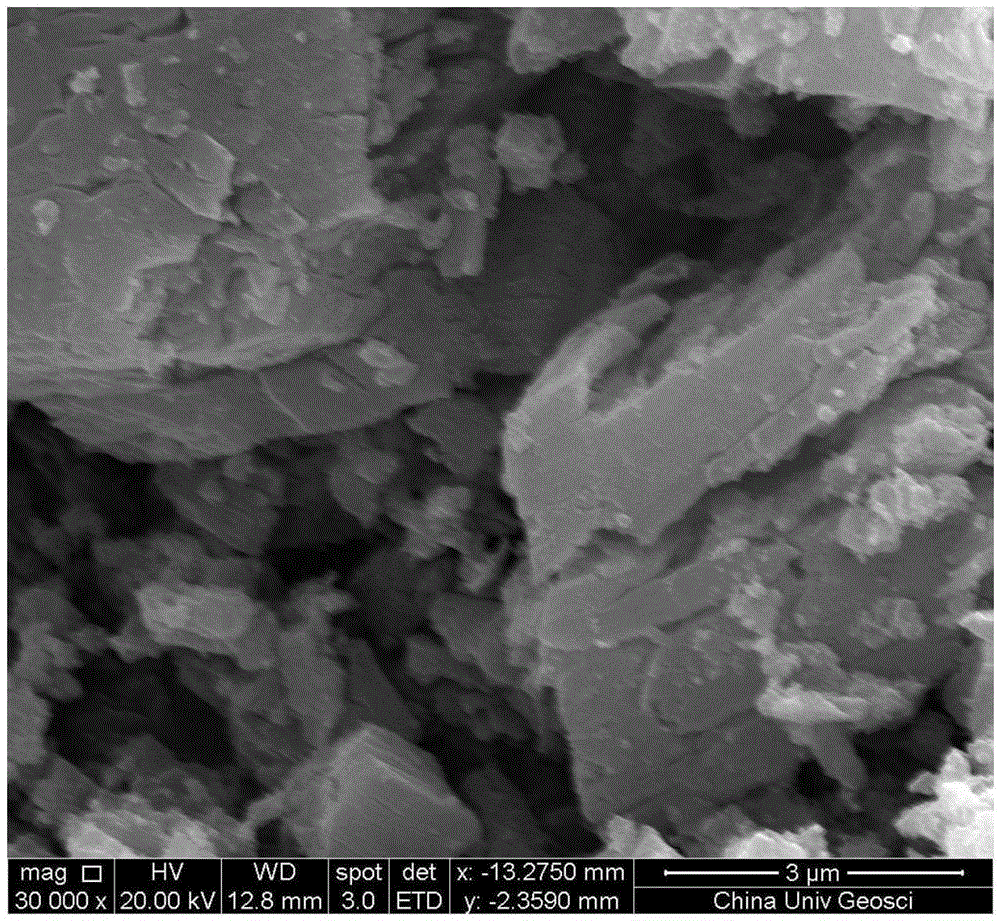
[0037] The electron scanning elect...
Embodiment 2
[0039] A sulfur-modified porous iron oxide catalyst is prepared by the following method: (1) Dissolving 0.02 mol of oxalic acid in 50 mL of water to prepare an oxalic acid solution, heating it to 60° C. in a water bath, and performing magnetic stirring during the heating process to make the oxalic acid completely (2) Under magnetic stirring, add 0.01 mol of sodium thiosulfate to the above-mentioned oxalic acid solution, stir to dissolve the sodium thiosulfate completely, and add dropwise 50 mL containing 0.02 mol Fe(NO) after mixing evenly. 3 ) 2 The solution obtained was a yellow suspension, and the reaction was continued to be stirred for 10 minutes, followed by an ice bath for 10 minutes; (3) the above-mentioned yellow suspension was vacuum filtered and washed, and then dried in a blast drying oven at 100 ° C until the water was dried to obtain The yellow granular sulfur-modified ferrous oxalate precursor was calcined in a muffle furnace and heated to 300 °C for 60 min at a...
Embodiment 3
[0045] A sulfur-modified porous iron oxide catalyst is prepared by the following method: (1) Dissolving 0.02 mol of oxalic acid in 50 mL of water to prepare an oxalic acid solution, heating it to 60° C. in a water bath, and performing magnetic stirring during the heating process to make the oxalic acid completely (2) Under magnetic stirring, add 0.01 mol of sodium thiosulfate to the above-mentioned oxalic acid solution, stir to dissolve the sodium thiosulfate completely, and add 50 mL of Fe(NO) containing 0.02 mol dropwise after mixing evenly. 3 ) 2 The solution obtained was a yellow suspension, and the reaction was continued to be stirred for 10 minutes, followed by an ice bath for 10 minutes; (3) the above-mentioned yellow suspension was vacuum filtered and washed, and then dried in a blast drying oven at 100 ° C until the water was dried to obtain The yellow granular sulfur-modified ferrous oxalate precursor was calcined in a muffle furnace, and heated to 300°C at a heating...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com