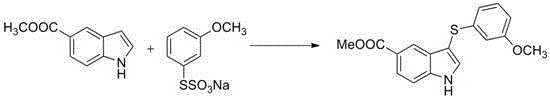Preparation method of 3-indole thioether
A technology of indole sulfide and indole, which is applied in the direction of organic chemistry, can solve the problems of human injury, narrow application range, and strong odor, and achieve the effects of mild reaction conditions, wide application range, and simple post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the preparation of 3-(3-methoxyphenylthio)indole
[0024]
[0025] To a 25 mL reaction flask was added indole (46.8 mg, 0.4 mmol), S-m-methoxybundt salt (242 mg, 1 mmol, 2.5 eq), elemental iodine (20 mg, 0.08 mmol), dimethyl sulfoxide ( 3mL). The reaction system was stirred and heated to 80 o C reacted for 12h. After the reaction, the reaction solution was diluted and washed with saturated sodium thiosulfate solution, and the resulting mixture was extracted four times with dichloromethane. The organic phases were combined, washed twice with water, and dried over anhydrous sodium sulfate. Finally, the solvent was evaporated, separated and purified by silica gel column chromatography (petroleum ether: ether / 20:1-4:1), and the final product 3-(m-methoxyphenylthio)indole was obtained as a white solid, (90mg, produced rate 88%). Melting point: 88-90 o c. 1 HNMR (CDCl 3 ,400MHz,ppm):δ8.42(brs,1H),7.63(d,J=8.0Hz,1H),7.41-7.46(m,2H),7.27-7.29(m,1H),7.16-...
Embodiment 2
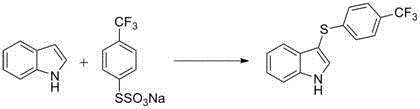
[0026] Embodiment 2: the preparation of 3-phenylthioindole
[0027]
[0028] The experimental operation was similar to Example 1, except that the amount of dimethyl sulfoxide was 78 mg (2.5 eq.), and DMF (3 mL) was used as the solvent to obtain 3-phenylthio-indole with a yield of 84%. White solid, melting point: 152-153 o c. 1 HNMR (CDCl 3,400MHz,ppm):δ8.37(brs,1H),7.37(d, J= 7.6Hz , 1H),7.43-7.48(m,2H),7.27-7.31(t, J= 7.2Hz , 1H), 7.05-7.20 (m, 6H). 13 CNMR (CDCl 3 ,100MHz,ppm):δ139.3,136.6,130.8,129.8,128.8,126.0,124.7,123.2,121.0,119.8,111.7,102.9.HRMS(ESI):m / zcalcdforC 14 h 11 NS[M+H] + ,226.0690;found,226.0693.
Embodiment 3
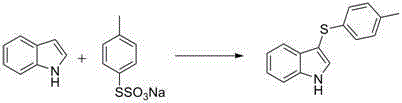
[0029] Embodiment 3: the preparation of 3-(4-methylphenylthio)-indole
[0030]
[0031] The experimental operation is similar to Example 1, except that the amount of dimethyl sulfoxide is 78 mg (2.5 eq.), and NMP (3 mL) is used as a solvent to obtain 3-(4-methylphenylthio) indole, and the yield is 88%. White solid, melting point: 124-126 o C.1HNMR (CDCl 3 ,400MHz,ppm):δ8.35(brs1H),7.63(d,J=8.0Hz,1H),7.47(s,1H),7.42-7.44(d,J=8.4Hz,1H),7.25-7.29( m,1H),7.17(t,J=7.6Hz,1H),7.04(d,J=8.0Hz,2H),6.98(d,J=8.4Hz,2H).2.26(s,3H). 13 CNMR (CDCl 3 ,100MHz,ppm):δ136.5,135.6,134.8,130.6,129.6,129.2,126.4,123.1,120.9,119.8,111.7,21.0.HRMS(ESI):m / zcalcdforC 15 h 13 NS[M+H] + ,240.0847; found, 240.0845.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com