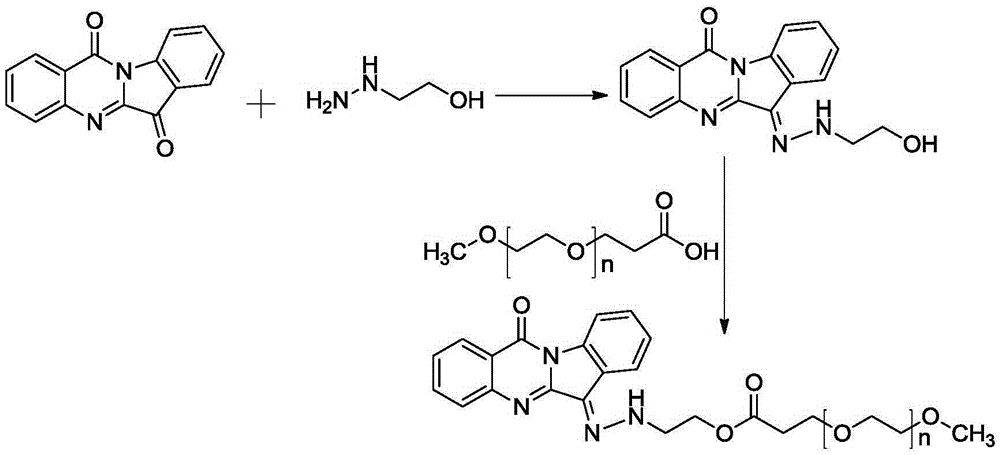
Polyethylene-glycol-modification water-solubility tryptanthrin polymer derivative, preparing method thereof and application thereof
A technology for polyethylene glycol and tryptamine, which is applied in the field of water-soluble tryptamine polymer derivatives and the preparation thereof, can solve the problems of improving the water solubility of tryptamine, and achieves improved bioavailability, improved water solubility, and improved water solubility. The effect of solubility enhancement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
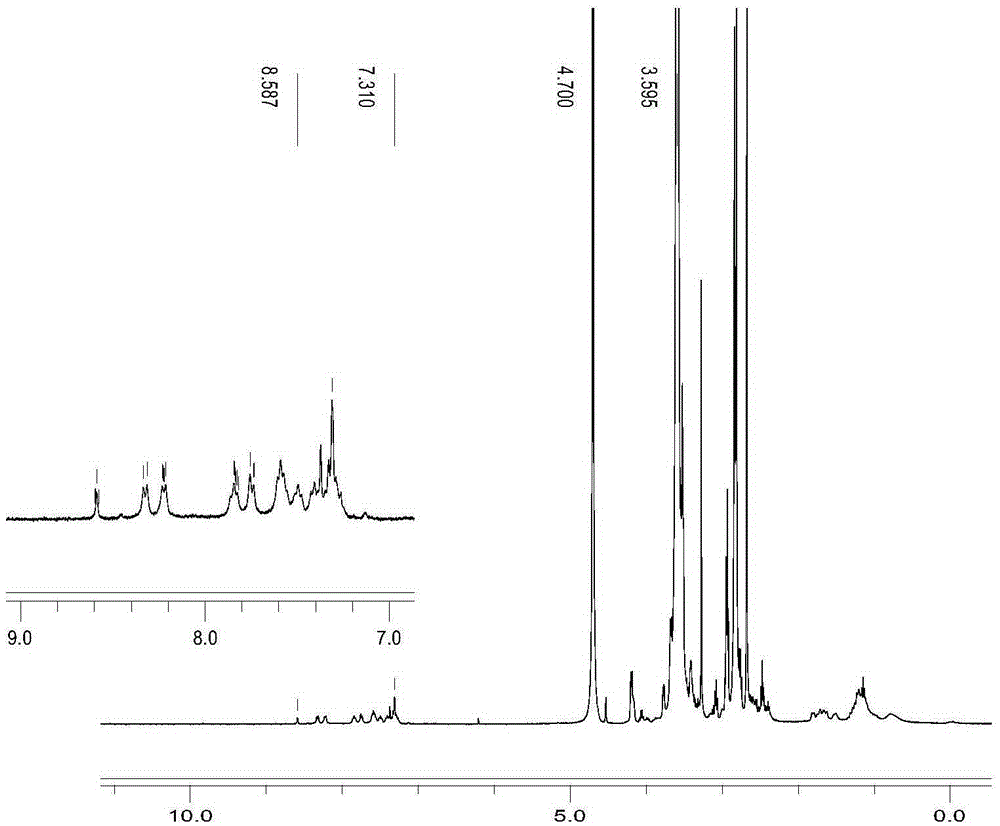
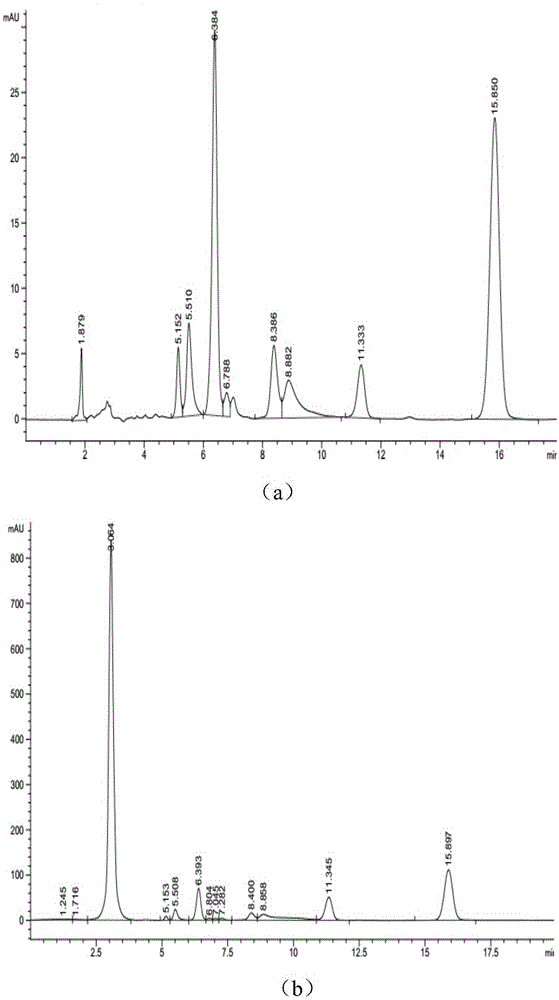
[0049] The synthetic route is attached figure 1 , 0.248g tryptanthrin was dissolved in 20mL tetrahydrofuran, 0.328g hydroxyethylhydrazine was dissolved in 2mL ethanol solution, vacuumed for 20min, protected by nitrogen, and stirred at room temperature for 12h. After the reaction, purify by column chromatography to collect the target product—TRYP-NNHCH 2 CH 2 Oh. The qualitative characterization of the fracture of its hydrazone bond is shown in the attached image 3 , wherein, (a) is pH7.4, t=15min; (b) is pH5.0, t=3min; (c) is pH5.0, t=30min.
[0050] 41.5mgmPEG 2000 -COOH, 12mgDCC and 6mgDMAP were dissolved in an appropriate amount of anhydrous dichloromethane, and reacted in an ice-water bath for 24h. Add appropriate amount of intermediate to mPEG 2000 -COOH solution, add 2 mL of pyridine at the same time, and continue to keep the ice-water bath for 24 hours. After the reaction, remove the solvent under reduced pressure, add saturated sodium bicarbonate solution, filte...
Embodiment 2
[0053] Dissolve 0.248g tryptanthrin in 15mL tetrahydrofuran, add 1.5mL hydrazine hydrate, 50°C oil bath, react for 10h, and purify by column chromatography to obtain the target product—TRYP-NNH 2 . 63.4mg of MPEG-COOH, 18.3mg of DDC, and 20.6mg of NHS were dissolved in anhydrous dichloromethane and reacted in an ice bath for 24 hours to obtain the activated product of mPEG-COOH. The activated product of the intermediate and mPEG-COOH was dissolved in anhydrous dichloromethane and reacted at room temperature for 24h. Rotary evaporation, saturated NaHCO 3 The solution was washed three times, extracted with ethyl acetate, and the product—TRYP-NNHCO-PEG was collected.
Embodiment 3
[0055] Weigh 100mgmPEG-OH and 13.1mg carbonyldiimidazole (CDI) into a 25mL eggplant-shaped bottle, add 5mL anhydrous dichloromethane, and stir at room temperature for 24 hours. After the reaction is completed, evaporate the solvent under reduced pressure, and add excess anhydrous water and ether, the white precipitate was collected and dried in vacuo to obtain 36.7 mg of mPEG-CDI. The product was dissolved in 5 mL of dichloromethane with hydrazine hydrate and triethylamine. The post-experiment processing is the same as the above step to obtain mPEG-NHNH 2 . This reacts with an equivalent of tryptanthrin to give TRYP-NNH-mPEG.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com