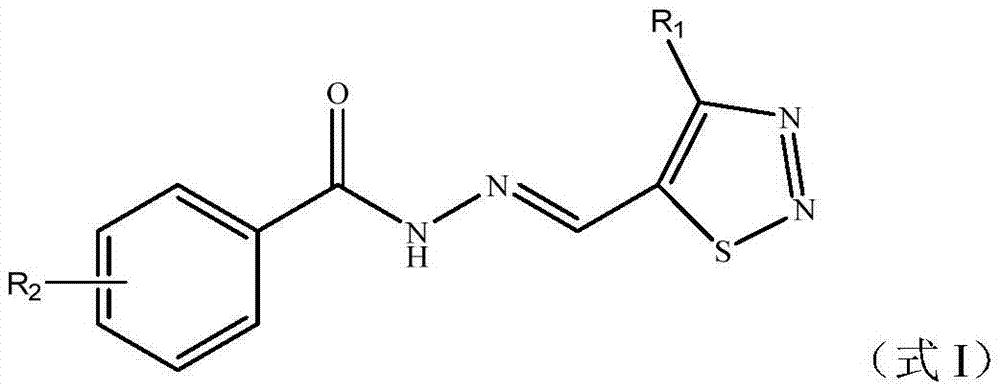
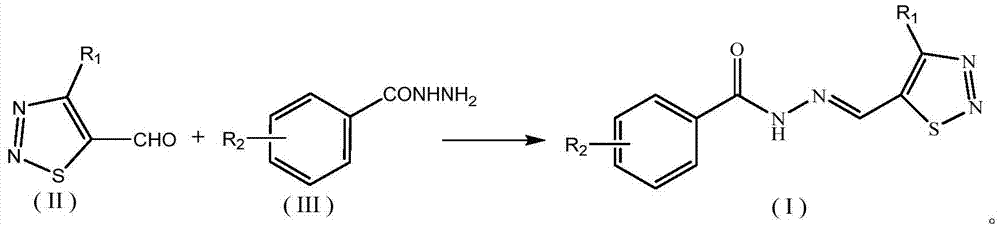
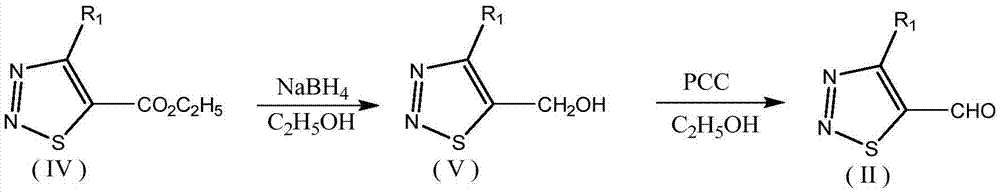
1,2,3-thiadiazole-containing benzoyl hydrazone derivative as well as preparation method and application thereof
A technology for benzoylhydrazone derivatives, applied in the field of organic chemical synthesis, can solve the problems of rare research literature on benzoylhydrazone derivatives, and achieve the effects of high inhibitory activity, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Preparation and structure identification of 4-butyl-1,2,3-thiadiazole-5-methanol (V-05)
[0037] Weigh 8.56g (0.04mol) of ethyl 4-butyl-1,2,3-thiadiazole-5-carboxylate into a 100ml three-neck flask, add 30mL of ethanol to dissolve, then place in an ice-salt bath and cool to 0°C Next, slowly add 2.28g (0.06mol) sodium borohydride, remove the ice-salt bath and heat up to 25°C, react at room temperature for 5 hours, add dilute hydrochloric acid to the reaction system until the solution is acidic, extract with dichloromethane, and use Wash with saturated sodium bicarbonate solution and saturated sodium chloride solution, dry the organic phase with anhydrous sodium sulfate, filter with suction, and concentrate the filtrate to obtain 6.31 g of yellow liquid, which is the intermediate V-05 of the present invention, with a yield of 91.7%.
[0038] V-01~V-04 were successfully prepared according to the same method as that used to prepare compound V-05. The physical and...
Embodiment 2
[0039]Example 2: Preparation and structure identification of intermediate 4-butyl-1,2,3-thiadiazole-5-carbaldehyde (II-05)
[0040] Weigh 10.63g (0.05mol) of PCC into a 100ml three-neck flask, add 40ml of dichloromethane and stir to dissolve, then slowly add 6.02g (0.035mol) of 4-butyl-1,2,3-thiadiazole-5-methanol The dichloromethane solution was monitored by TLC until the reaction was complete. Stop the reaction, filter with suction, wash the filtrate with saturated sodium bicarbonate solution and saturated sodium chloride solution respectively, dry the organic phase with anhydrous sodium sulfate, filter with suction, and concentrate the filtrate to obtain 5.39 g of yellow liquid, which is intermediate II of the present invention -05, yield 90.6%.
[0041] According to the same method as the preparation of compound II-05, II-01~II-04 were successfully prepared, and their physical and chemical data and structural identification data are shown in Table 1. From the relevant dat...
Embodiment 3
[0046] Example 3: Preparation and structure identification of 4-methyl-1,2,3-thiadiazole-5-carbaldehyde-2-bromo-benzoylhydrazone (No. I-01)
[0047] Add 20mL of methanol and 0.32g (2.5mmol) of the compound 4-methyl-1,2,3-thiadiazole-5-carbaldehyde shown in formula II to a 50mL three-necked flask, stir, and add 0.6mg (0.01mmol) in one go Acetic acid is activated, and then the compound shown in 0.43g (2mmol) formula III (R 2 2-substituted bromo) 2-bromo-benzohydrazide was dissolved in methanol, slowly added to the above-mentioned three-necked flask, heated to 78°C and refluxed for 6 hours to stop the reaction. The solvent was removed by rotary evaporation to obtain a crude product, which was recrystallized from ethanol to obtain 0.56 g of a white solid with a yield of 87%. The appearance and fusing point of this white solid product are shown in Table 2, and its 1 HNMR spectrum data are shown in Table 3. It can be seen from Table 3 that the product has a correct structure and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com