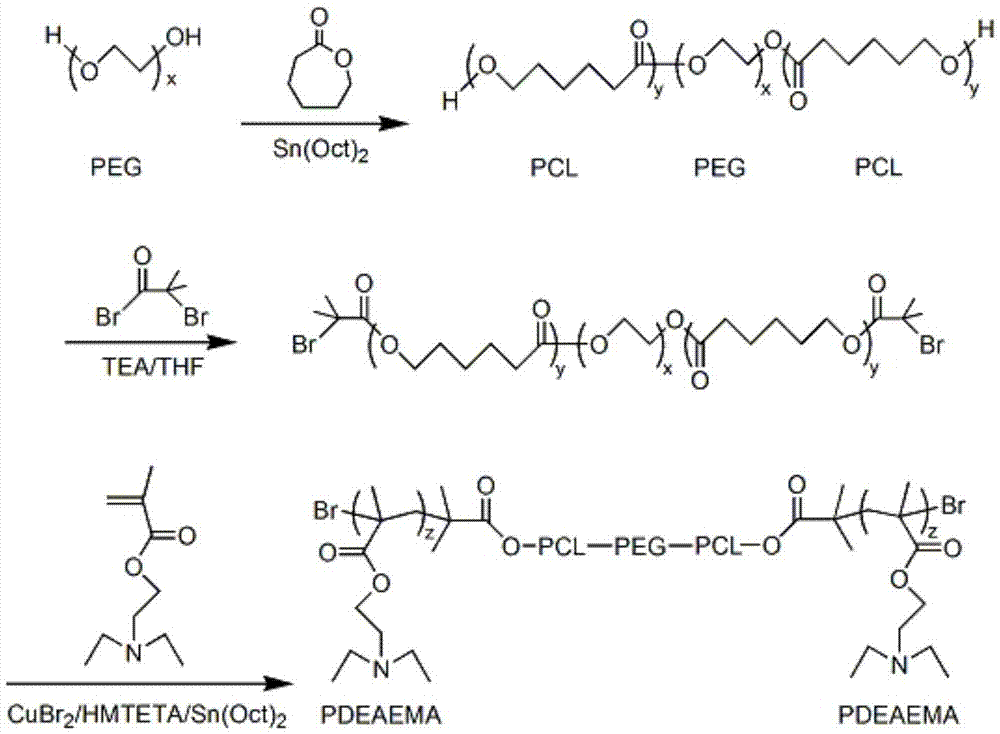
pH/temperature sensitive amphiphilic polymer, and preparation method and applications thereof
An amphiphilic copolymer, dual-sensitivity technology, applied in non-active ingredients medical preparations, active ingredients-containing medical preparations, drug combinations, etc., can solve problems such as normal tissue and cell damage, and achieve cell-free Toxicity, avoidance of residues, and the effect of improving degradation performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1: Preparation of five-block copolymer PDEAEMA-b-PCL-b-PEG-b-PCL-b-PDEAEMA
[0063] Synthetic reaction formula see figure 1 .
[0064] (1) Synthesis of triblock copolymer PCL-b-PEG-b-PCL
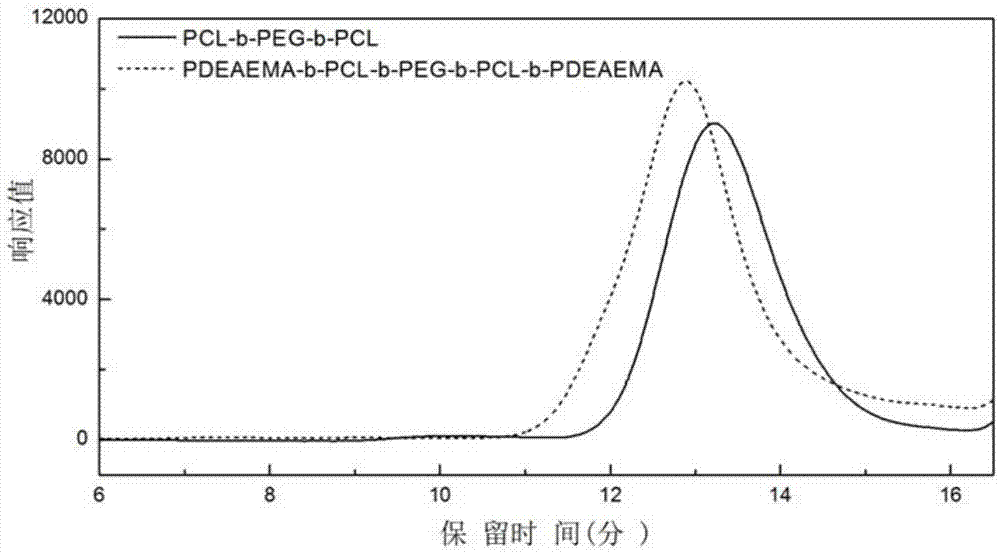
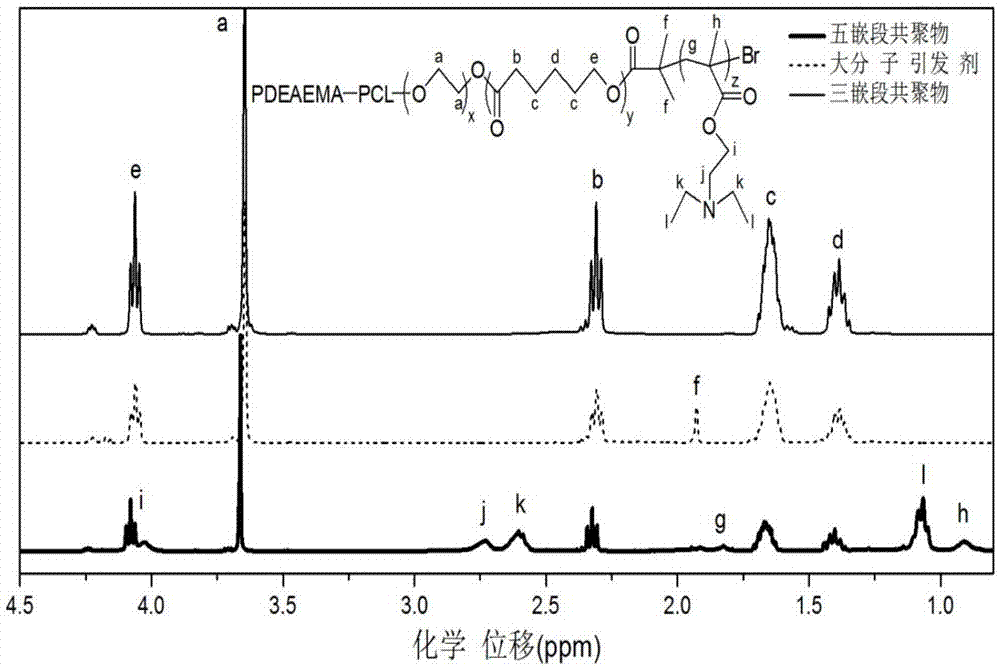
[0065] Take a 100mL reaction bottle, put it into a stirring bar, mix PEG (Mn=1000, 20g, 0.02mol) and Sn(Oct) 2 (0.4g, 0.001mol) was added to the reaction bottle and sealed with a reverse rubber stopper. Vacuum-puff argon for 3 times, add monomeric caprolactone (38.07mL, 0.36mol) under the protection of argon, and perform three cycles of freezing-pumping-heating with liquid nitrogen, under the protection of argon, at 130°C Under reaction 48h. After the reaction, cool down, dissolve the solid with 50mL THF, precipitate with 500mL ether at 0°C, filter with suction, and vacuum-dry the precipitated product at 35°C and 3.5kPa for 48h to obtain a triblock copolymer. Its molecular weight was measured by gel permeation chromatography (GPC), and its structure was analyzed by hyd...
Embodiment 2
[0071] Example 2: Preparation of Pentablock Copolymer PDEAEMA-b-PCL-b-PEG-b-PCL-b-PDEAEMA
[0072] Synthetic reaction formula see figure 1 .
[0073] (1) Synthesis of triblock copolymer PCL-b-PEG-b-PCL
[0074] Take a 100mL reaction bottle, put it into a stirring bar, mix PEG (Mn=1000, 20g, 0.02mol) and Sn(Oct) 2(0.8g, 0.002mol) was added to the reaction bottle and sealed with a reverse rubber stopper. Vacuumize - argon for 3 times, add monomer caprolactone (55.66mL, 0.53mol) under the protection of argon. Under reaction 56h. After the reaction, cool down, dissolve the solid with 50mL THF, precipitate with 500mL ether at 0°C, filter with suction, and vacuum-dry the precipitated product at 35°C and 3.5kPa for 48h to obtain a triblock copolymer. Gel permeation chromatography (GPC) elution curves of triblock copolymers and figure 2 Similar, wherein Mn=3885, Mw / Mn=1.28; The proton nuclear magnetic resonance spectrum figure and image 3 unanimous.
[0075] (2) Synthetic ma...
Embodiment 3
[0079] Example 3: Preparation of Pentablock Copolymer PDEAEMA-b-PCL-b-PEG-b-PCL-b-PDEAEMA
[0080] Synthetic reaction formula see figure 1 .
[0081] (1) Synthesis of triblock copolymer PCL-b-PEG-b-PCL
[0082] Take a 100mL reaction bottle and put it into a stirring bar. PEG (Mn=1000, 20g, 0.02mol) and Sn(Oct) 2 (0.8g, 0.002mol) was added to the reaction bottle and sealed with a reverse rubber stopper. Vacuum-puff argon for 3 times, add monomeric caprolactone (38.07mL, 0.36mol) under the protection of argon, and perform three cycles of freezing-pumping-heating with liquid nitrogen, under the protection of argon, at 140°C Under reaction 56h. After the reaction, cool down, dissolve the solid with 50mL THF, precipitate with 500mL ether at 0°C, filter with suction, and vacuum-dry the precipitated product at 35°C and 3.5kPa for 48h to obtain a triblock copolymer. Gel permeation chromatography (GPC) elution curves of triblock copolymers and figure 2 Similar, wherein Mn=2903,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com