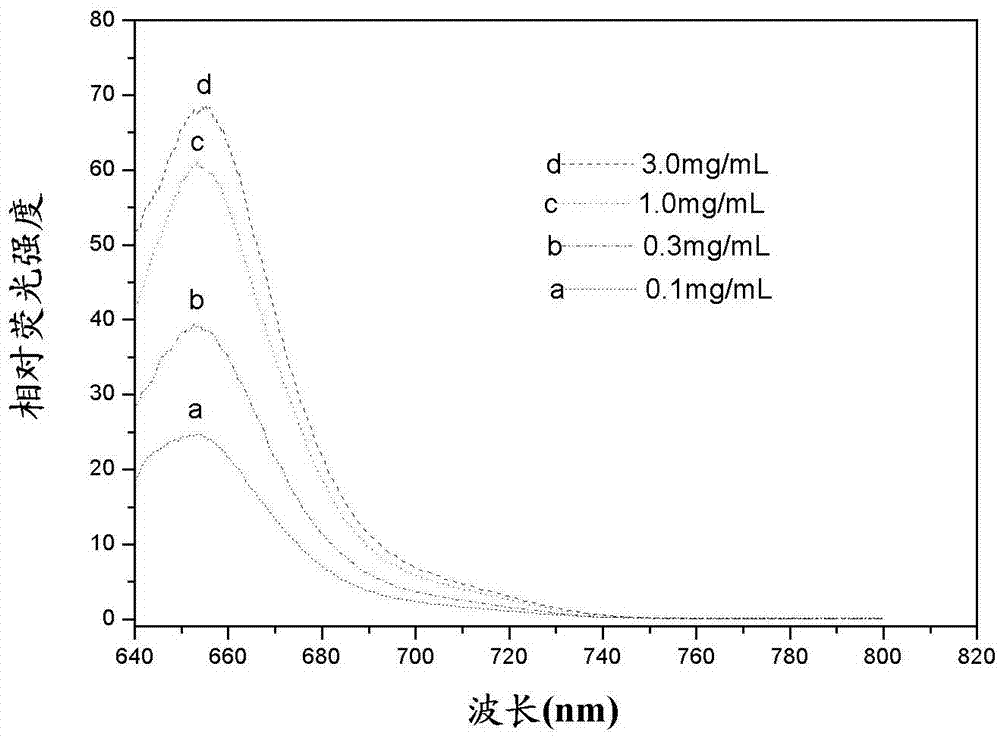
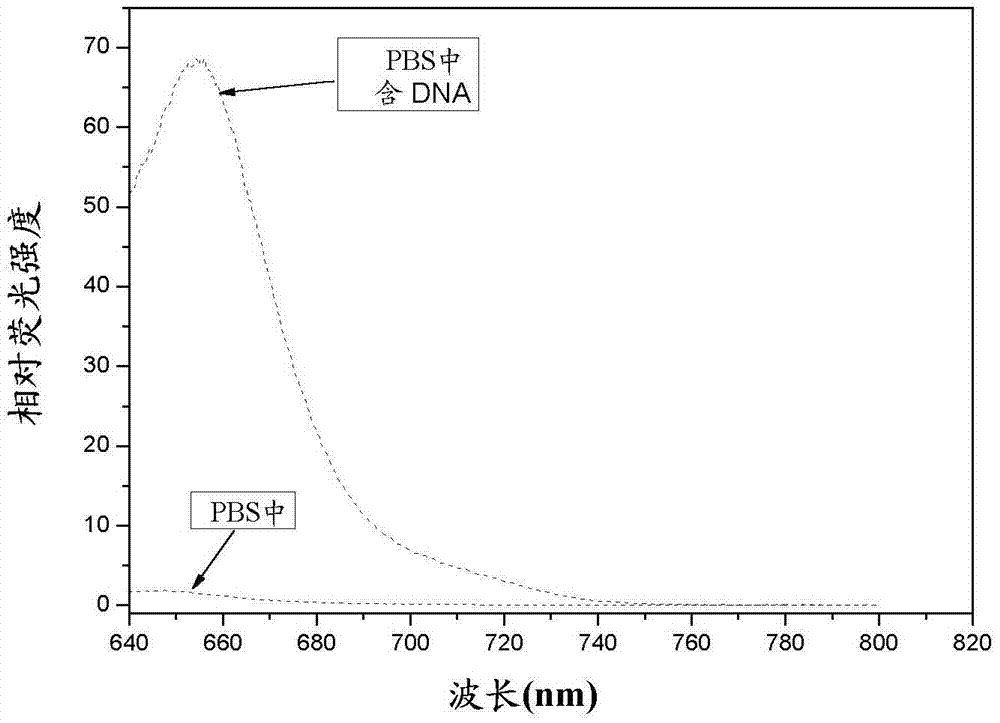
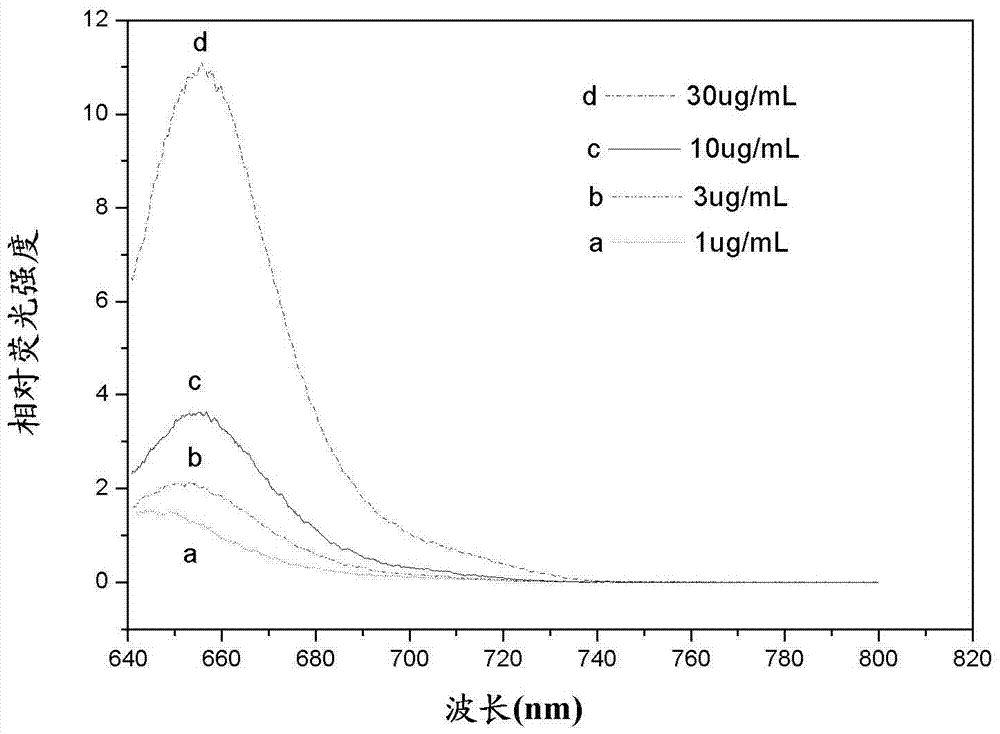
Asymmetric cyanine fluorescent dye, composition and application in biological sample dyeing
A technology for fluorescent dyeing and biological samples, applied in the field of asymmetric cyanine fluorescent dyes, can solve the problems of long synthesis route, limited scope of use, complex molecular structure, etc., and achieve the effect of improving accuracy and avoiding fluorescence background interference.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0064] The preparation method of the compound of the present invention
[0065] The compound of the present invention can be synthesized by general methods well known in the art, see for example the co-pending Chinese patent application CN200710137258.6 belonging to the present applicant. Said patent application is incorporated herein by reference. Specifically, the asymmetric cyanine fluorescent dyes of the present invention are generally synthesized by the following process: first, unsubstituted or substituted 2-methylbenzothiazole, 2-methylbenzothiazole or 2,3,3-tri Starting from raw materials such as methyl-3H-indoline, making it with the formula R 3 X (X is F, Cl, Br or I) halide reaction, the molar ratio of the two is 1:1-2, refluxed in toluene for 12-36 hours, the quaternary ammonium salt intermediate II can be obtained:
[0066]
[0067] where X, R 1 , R 3 and Y - as defined in the compound of formula I;
[0068] Then, the prepared quaternary ammonium salt int...
Embodiment 1
[0090] Synthesis of Dye-1
[0091]
[0092] In a mixed solution of 40mL methanol and 20mL ethanol, heat and stir 10mmol 1-hexanoic acid ethyl-2-methylbenzothiazole bromide quaternary ammonium salt and 30mmol N,N'-diphenylformamidine on an oil bath at 65°C 6 hours. After the reaction, the solvent was distilled off under reduced pressure, and then a certain amount of diethyl ether was added and stirred to precipitate an orange solid powder, which was filtered and dried. The crude product was recrystallized from ethyl acetate-hexane to obtain an orange-red solid product with a yield of 42%. Take 4.0 mmol of the reaction product, add 4.2 mmol of 1-benzyl-4-methylquinoline quaternary ammonium salt and 10 ml of pyridine, and heat and stir on an oil bath at 90° C. for 1.5 hours. After cooling to room temperature, the reaction solution was poured into ether, and a dark purple solid was precipitated, which was filtered and dried. The dye was separated through a silica gel colum...
Embodiment 2
[0095] Synthesis of Dye-2
[0096]
[0097] In a mixed solution of 40mL methanol and 20mL ethanol, put 10mmol 1-(2-hydroxyethyl)-2-methylbenzothiazole bromide quaternary ammonium salt and 30mmol N,N'-diphenylformamidine on an oil bath at 65°C Heat and stir for 6 hours. After the reaction, the solvent was distilled off under reduced pressure, and then a certain amount of diethyl ether was added and stirred to precipitate an orange solid powder, which was filtered and dried. The crude product was recrystallized from ethyl acetate-hexane to obtain an orange-red solid product with a yield of 41%. Take 4.0 mmol of the reaction product, add 4.5 mmol of 1-(ethylbutyrate)-4-methylquinoline quaternary ammonium salt and 10 ml of pyridine, and heat and stir on an oil bath at 90° C. for 1.5 hours. After cooling to room temperature, the reaction solution was poured into ether, and a purple-red solid was precipitated, which was filtered and dried. The dye was separated through a sil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com