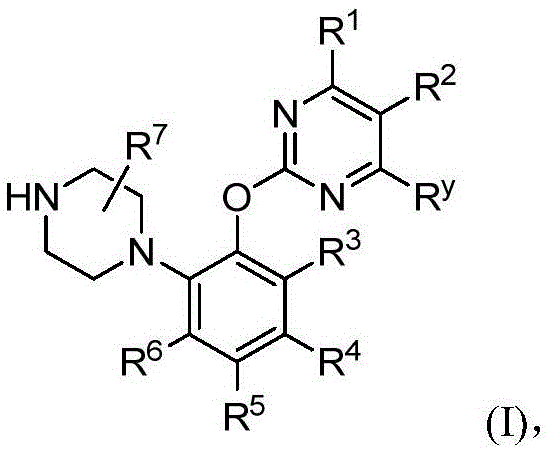
Phenylpiperazine derivatives, using method thereof and uses of the derivatives
An alkyl and drug technology, applied to compounds and compositions for affective disorders, in the field of treatment of central nervous system dysfunction, can solve problems such as deterioration and delayed treatment of SSRIs, and achieve stable properties, good safety, and good metabolic stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
[0152] Example 14,6-dimethoxy-2-(2-(piperazin-1-yl)phenoxy)pyrimidine
[0153]
[0154] Step 1) Synthesis of tert-butyl 4-(2-((4,6-dimethoxypyrimidin-2-yl)oxy)phenyl)piperazine-1-carboxylate
[0155] 4-(2-hydroxyphenyl)piperazine-1-carboxylic acid tert-butyl ester (0.42g, 1.51mmol), 2-chloro-4,6-dimethoxypyrimidine (0.28g, 1.60mmol) and potassium carbonate (0.42g, 3.02mmol) was sequentially added to DMSO (10mL), and then the reaction solution was heated to 110°C for 20 hours. After the reaction, the reaction solution was cooled to room temperature, washed with water (20 mL), and extracted with ethyl acetate (20 mL×3). The combined organic phases were dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column purification (petroleum ether / ethyl acetate (v / v)=2 / 1) to obtain the title compound as a pale yellow solid (0.44 g, 70.1%).
[0156] MS(ESI,pos.ion)m / z:417.2[M+H] + ;
...
Embodiment 24
[0162] Example 24,6-Dimethyl-2-(2-(piperazin-1-yl)phenoxy)pyrimidine
[0163]
[0164] Step 1) Synthesis of tert-butyl 4-(2-((4,6-dimethylpyrimidin-2-yl)oxy)phenyl)piperazine-1-carboxylate
[0165] The title compound of this step can be prepared by referring to the method described in step 1 of Example 1, that is, tert-butyl 4-(2-hydroxyphenyl)piperazine-1-carboxylate (0.42g, 1.51mmol), 2-chloro-4 , 6-dimethylpyrimidine (0.23g, 1.60mmol) and potassium carbonate (0.42g, 3.02mmol) were reacted in DMSO (10mL) to prepare crude product, which was purified by silica gel column (petroleum ether / ethyl acetate ( v / v) = 2 / 1) to obtain the title compound as a pale yellow solid (0.39 g, 67.2%).
[0166] MS(ESI,pos.ion)m / z:385.2[M+H] + ;
[0167] 1 HNMR (CDCl 3 ,400MHz)δ(ppm):7.21-7.17(m,2H),7.12-7.08(m,2H),6.73(s,1H),3.19-3.17(m,4H),2.90-2.88(m,4H) ,2.36(s,6H),1.44(s,9H).
[0168] Step 2) Synthesis of 4,6-dimethyl-2-(2-(piperazin-1-yl)phenoxy)pyrimidine
[0169] Dissolve ...
Embodiment 34
[0172] Example 34,6-dichloro-2-(2-(piperazin-1-yl)phenoxy)pyrimidine
[0173]
[0174] Step 1) Synthesis of 4-(2-((4,6-dichloropyrimidin-2-yl)oxy)phenyl)piperazine-1-carboxylic acid tert-butyl ester
[0175] Under nitrogen protection, tert-butyl 4-(2-hydroxyphenyl)piperazine-1-carboxylate (0.42g, 1.51mmol), 4,6-dichloro-2-(methylsulfonyl)pyrimidine (0.36g, 1.60mmol) and potassium carbonate (0.42g, 3.02mmol) were added into dichloromethane (10mL), and the reaction solution was reacted at room temperature for 12 hours. After the reaction, the reaction solution was washed with water (20 mL×3), the combined organic phase was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column (petroleum ether / ethyl acetate (v / v)=2 / 1) to obtain the title compound as a pale yellow solid (0.32 g, 49.8%).
[0176] MS(ESI,pos.ion)m / z:426.2[M+H] + ;
[0177] 1 HNMR (CDCl 3 ,400MHz)δ(ppm):7.27-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com