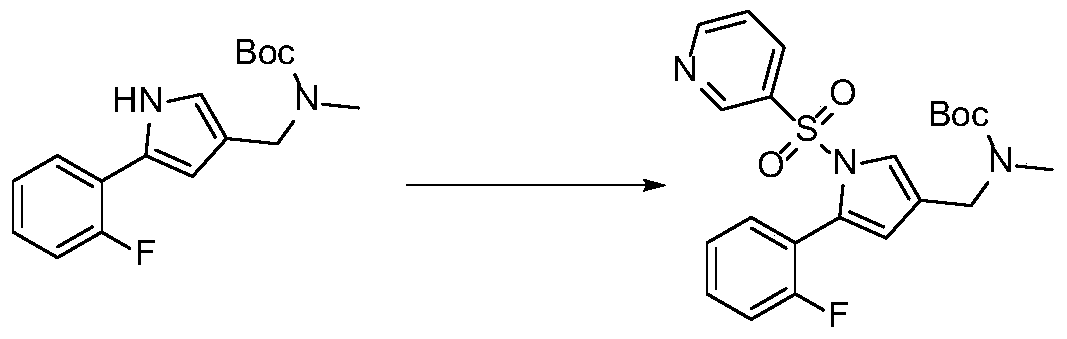
Preparation method of high-purity ((5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1h-pyrrol-3-yl)methyl)(methyl)carbamate tert-butyl ester
A technology of methyl carbamic acid and sulfonyl group, which is applied in the field of medicine, can solve the problems unfavorable to the industrial production of vonoprazan fumarate, and achieve the effects of avoiding post-processing methods, simple process and stable yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
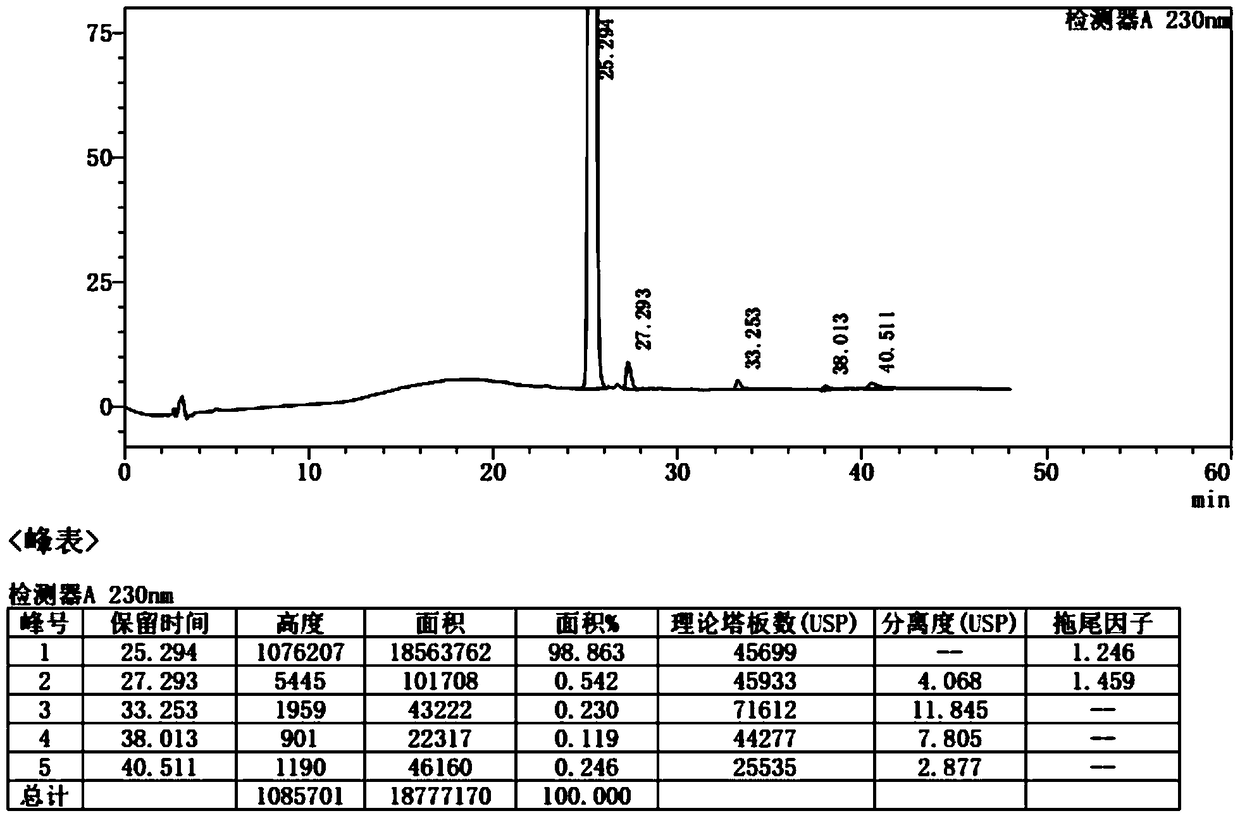
Image
Examples
Embodiment 1
[0023] S1: Synthesis of tert-butyl ((5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl)methyl)(methyl)carbamate
[0024] Take 60g of ((5-(2-fluorophenyl)-1H-pyrrol-3-yl)-N-methyl) methylcarbamate tert-butyl ester dissolved in 240ml of anhydrous tetrahydrofuran, nitrogen protection, slowly under ice bath Add 24g of sodium hydride in batches, stir in an ice bath for 10 minutes, heat up to 50-60°C for 0.2-2h, cool down to 0-5°C, slowly add 53.2g of 3-pyridinesulfonyl chloride anhydrous tetrahydrofuran solution 160ml dropwise, After the dropwise addition was completed, stir and react in an ice bath for 10 minutes, raise the temperature to 25-60° C., and stir and react for 1-3 hours. TLC confirmed the reaction end point. Solution of crude tert-butyl phenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl)methyl)(methyl)carbamate in THF.
[0025] S2: Purification of tert-butyl ((5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl)methyl)(methyl)carbamate
[0026] Add 1200m...
Embodiment 2
[0028] S1: Synthesis of tert-butyl ((5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl)methyl)(methyl)carbamate
[0029] Take 60g of ((5-(2-fluorophenyl)-1H-pyrrol-3-yl)-N-methyl) methylcarbamate tert-butyl ester dissolved in 250ml of anhydrous ether, nitrogen protection, slowly under ice bath Add 28g of sodium hydride in batches, stir in an ice bath for 10 minutes, heat up to 30-35°C and react for 30-45 minutes, cool down to 0-5°C, slowly add 53.2g of 3-pyridinesulfonyl chloride anhydrous ether solution 200ml, After the dropwise addition, stir and react in an ice bath for 10 minutes, rise to 30-50°C, stir and react for 1.5-2 hours, and determine the end point of the reaction by TLC. The developer is: V 石油醚 :V 乙酸乙酯 = 1:1, after the reaction is complete, add diatomaceous earth for suction filtration, wash the filter cake with 100ml of anhydrous ether, combine the filtrate and washings, extract with 500ml of purified water and 20% saline respectively, and dry the organ...
Embodiment 3
[0033] S1: Synthesis of tert-butyl ((5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl)methyl)(methyl)carbamate
[0034] Get 60g of ((5-(2-fluorophenyl)-1H-pyrrol-3-yl)-N-methyl)methylcarbamate tert-butyl ester dissolved in 240ml of anhydrous methyl tetrahydrofuran, nitrogen protection, ice bath Slowly add 24g of sodium hydride in batches, stir in an ice bath for 15 minutes, heat up to 60-70°C for 15-30 minutes, cool down to 0-5°C, and slowly add 53.2g of 3-pyridinesulfonyl chloride anhydrous methyl 150ml of tetrahydrofuran solution. After the dropwise addition, stir the reaction in an ice bath for 10 minutes, raise the temperature to 50-80°C, and stir the reaction for 1-3 hours. The end point of the reaction is determined by TLC. The filter cake was washed with tetrahydrofuran, the combined filtrate and washings were extracted with 500ml purified water and 20% saline respectively, and the organic phase was dried over anhydrous sodium sulfate to obtain ((5-(2-fluoroph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com