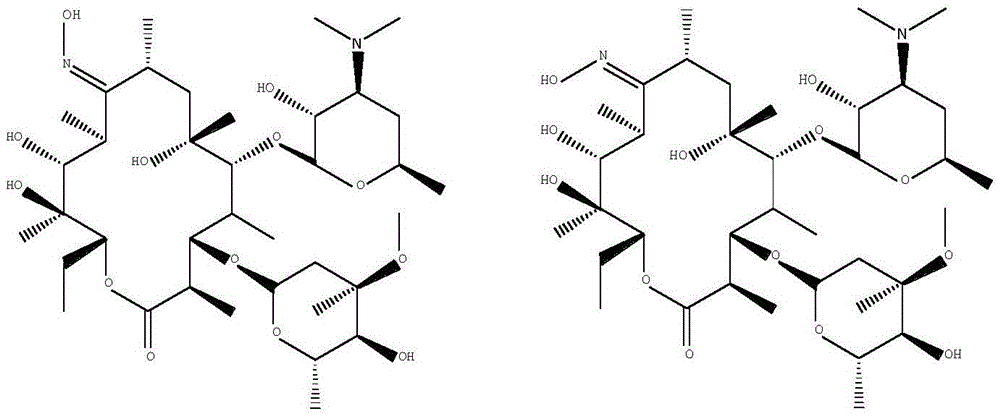
Synthesis method of 9-desoxy-9-homoerythromycin A(Z) oxime
A synthesis method and technology of erythromycin, applied in chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve problems such as affecting stable existence, inability to obtain products, yellow color, etc., and achieve less impurities and better operation. Simple, color-free effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A kind of synthetic method of 9-deoxy-9-homoerythromycin A (Z) oxime, its step is:
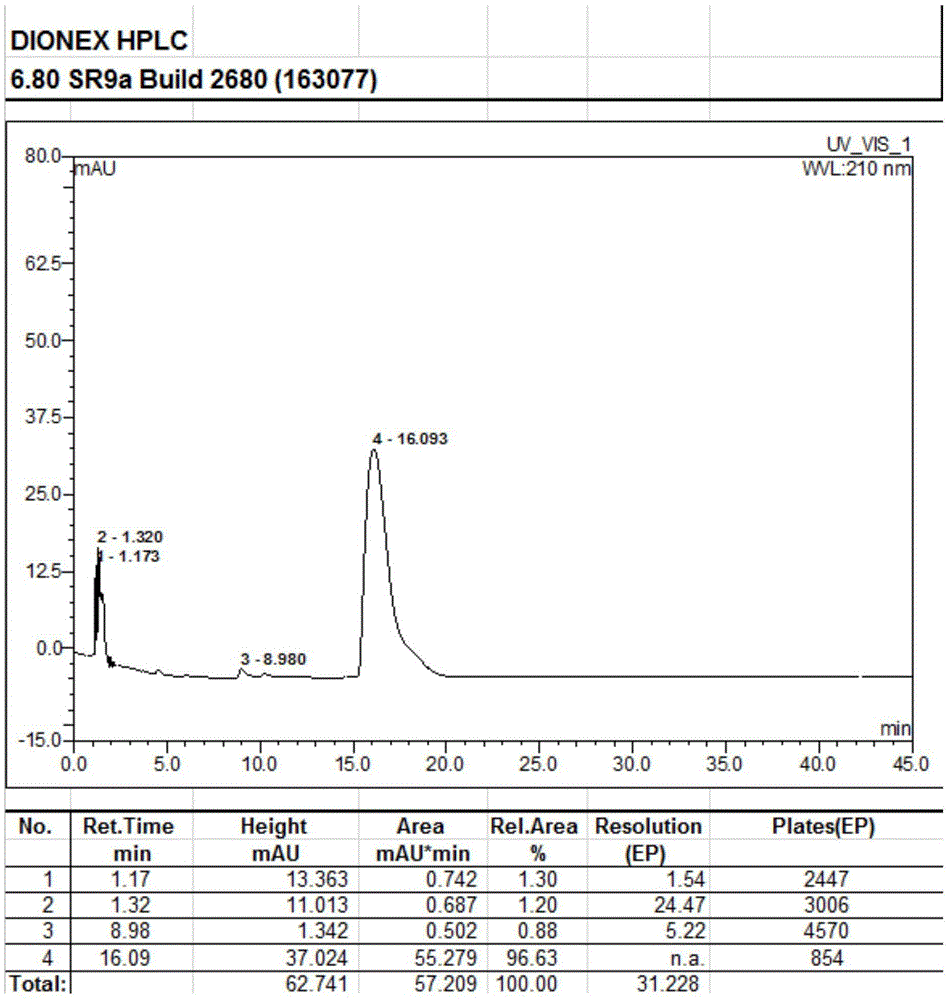
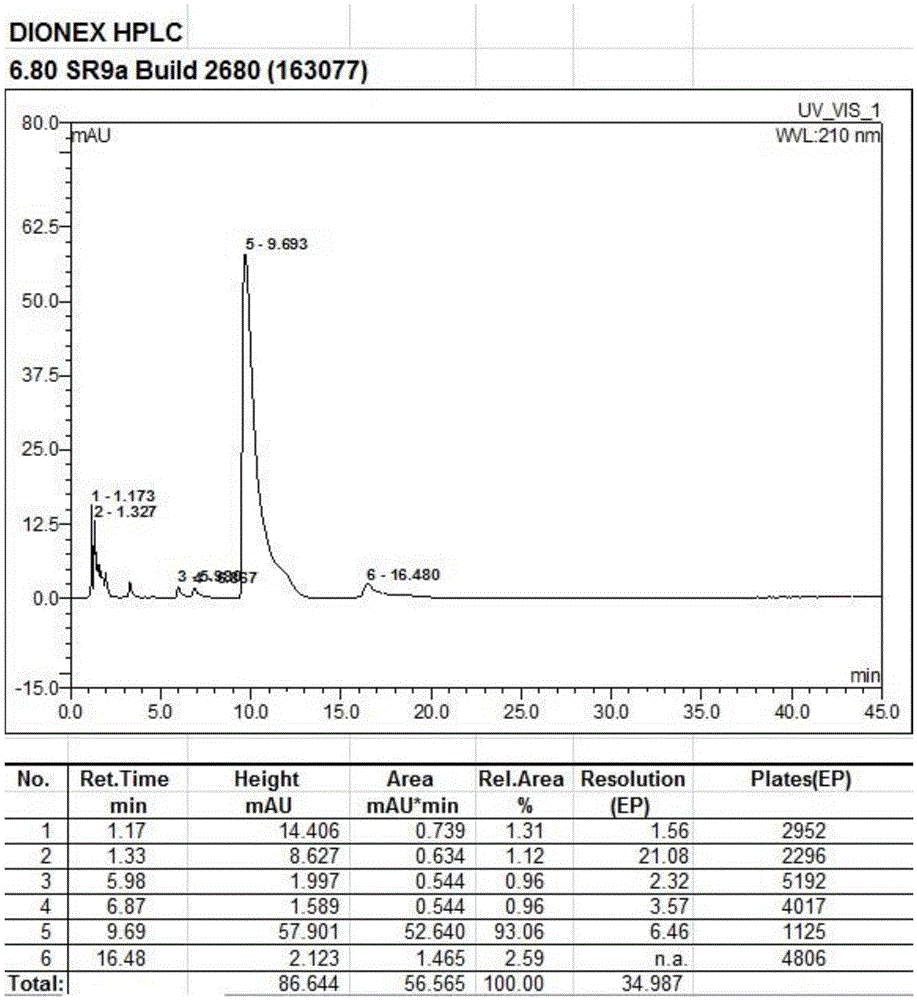
[0038] Add 100g of 9-deoxy-9-homoerythromycin A (E) oxime into a three-necked flask, then add 500ml of dichloromethane, stir until 9-deoxy-9-homoerythromycin A (E) oxime is completely dissolved, then add 6.8g of triethylamine was stirred at 3°C for 58 hours. After stirring for 54-57 hours, the reaction system changed from a clear transparent liquid to a milky emulsion, and continued to stir for 24 hours. At this time, the reaction was completed, filtered, and the obtained The filter residue was washed with 50ml of dichloromethane at 3°C to obtain a white solid, which was vacuum-dried at 40°C and -0.098Mpa for 5 hours to obtain 88.9g of 9-deoxy-9-homoerythromycin A(Z) oxime . The obtained 9-deoxy-9-homoerythromycin A(Z) oxime is a white solid with a melting point of 158.7°C-162.0°C (157-164°C in EP0503949), a purity of 93.06%, and a yield of 90.43%. The results of the infrared spec...
Embodiment 2
[0044] A kind of synthetic method of 9-deoxy-9-homoerythromycin A (Z) oxime, its step is:
[0045] Add 100g of 9-deoxy-9-homoerythromycin A (E) oxime into a three-necked flask, then add 500ml of ethyl acetate, stir until 9-deoxy-9-homoerythromycin A (E) oxime is completely dissolved, then add 4.92g of diethylamine, stirred at 0°C for 48 hours, after stirring for 46-47 hours, the reaction system changed from a clear liquid to a milky emulsion, and continued to stir for 24 hours. At this time, the reaction was completed, filtered, and the obtained The filter residue was washed with 50ml of ethyl acetate at 0°C to obtain a white solid, which was vacuum-dried at 40°C and -0.098Mpa for 5 hours to obtain 91.1g of 9-deoxy-9-homoerythromycin A(Z) oxime . The resulting 9-deoxy-9-homoerythromycin A(Z) oxime is a white solid with a melting point of 158.8°C to 162.3°C (EP0503949 is 157-164°C), a purity of 93.01%, and a yield of 87.69%. The result is: IR(KBr)σ, cm -1 : 3550 (N-OH), 1725...
Embodiment 3
[0049] A kind of synthetic method of 9-deoxy-9-homoerythromycin A (Z) oxime, its step is:
[0050] Add 100g of 9-deoxy-9-homoerythromycin A (E) oxime into a three-necked flask, then add 500ml of pentanone, stir until 9-deoxy-9-homoerythromycin A (E) oxime is completely dissolved, then add 2.69 g sodium hydroxide, stirred at 5°C for 40 hours, after stirring for 38-39 hours, the reaction system changed from a clear liquid to a milky emulsion, and continued to stir for 24 hours. At this time, the reaction was completed, filtered, and the obtained The filter residue was washed with 50ml of 5°C pentanone to obtain a white solid, which was vacuum-dried at 40°C and -0.098Mpa for 5 hours to obtain 87.1g of 9-deoxy-9-homoerythromycin A(Z) oxime. The obtained 9-deoxy-9-homoerythromycin A(Z) oxime is a white solid with a melting point of 158.5°C-162.2°C (157-164°C in EP0503949), a purity of 93.12%, and a yield of 83.94%. The results of the infrared spectrum are: IR(KBr)σ, cm -1 : 3550 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com