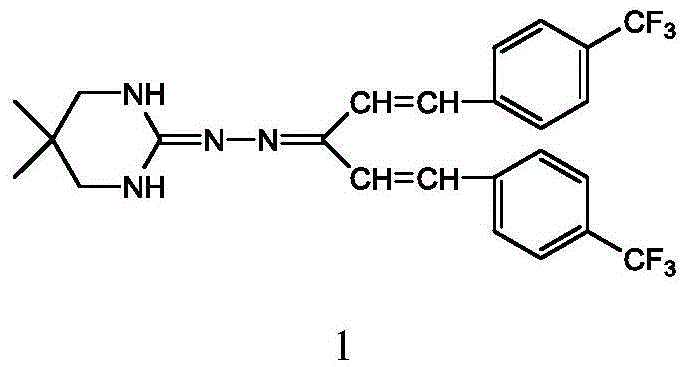
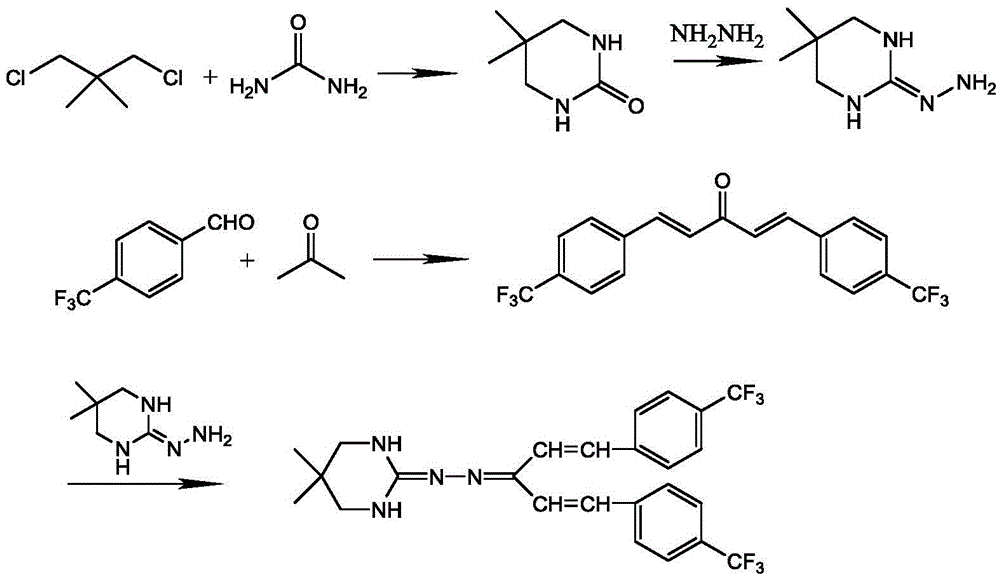
Synthetic process of hydramethylnon
A synthetic process, the technology of hydrazone, which is applied in the field of new synthetic technology of hydrazone, can solve the problems of unavailable, expensive catalysts, etc., and achieve the effects of mild reaction conditions, low cost and safe production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A kind of synthetic technique of embodiment 1 hydrazone
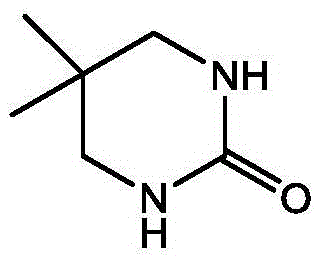
[0039] Step I. Synthesis of 5,5-dimethyl-1,4,5,6-tetrahydropyrimidin-2-one
[0040]
[0041] Add 70.5g (0.5mol) of 2,2-dimethyl-1.3-dichloropropane, 150ml of toluene, and 45g (0.75mol) of urea into a 500ml four-necked reaction flask, heat it, and add triethylamine dropwise under reflux Amine 75.6g (0.75mol), after the dropwise addition, continue to reflux for 4h; after the reaction, at 50°C, distill off the toluene under reduced pressure, add an appropriate amount of water to the residue, and extract twice with dichloromethane, 50ml each time , combined dichloromethane pages, washed with water, dried over magnesium sulfate, and concentrated to dryness to obtain 121.5 g of the title compound (white solid), yield 84%;
[0042] m / z:129[M+1] + .
[0043] Step II. Synthesis of 2-hydrazino-5,5-dimethyl-1,4,5,6-tetrahydropyrimidine hydrochloride
[0044]
[0045]Add 64.0g (0.5mol) of 5,5-dimethyl-1,4,5,6-tetra...
Embodiment 2
[0056] Step I. Synthesis of 5,5-dimethyl-1,4,5,6-tetrahydropyrimidin-2-one
[0057]
[0058] Add 70.5g (0.5mol) of 2,2-dimethyl-1.3-dichloropropane, 150ml of chloroform, and 36g (0.60mol) of urea into a 500ml four-necked reaction flask, heat it, and add triethylamine dropwise under reflux Amine 75.6g (0.75mol), after the dropwise addition, continue to reflux for 6h; after the reaction, add an appropriate amount of water, extract the water layer with chloroform twice, 50ml each time, combine the chloroform phases, wash with water, dry over magnesium sulfate, and concentrate to dryness The title compound 110g (white solid) was obtained with a yield of 76%;
[0059] m / z:129[M+1] + .
[0060] Step II. Synthesis of 2-hydrazino-5,5-dimethyl-1,4,5,6-tetrahydropyrimidine hydrochloride
[0061]
[0062] Add 64.0g (0.5mol) of 5,5-dimethyl-1,4,5,6-tetrahydropyrimidine- 2-ketone, 24g (0.6mo1) 80% hydrazine hydrate. Stir at room temperature until it becomes transparent, then heat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com