A kind of biphenylurea compound containing quinazolinone and its preparation method and application
A technology of quinazolinone and biphenylurea, applied in the field of biomedicine, can solve problems such as hair loss, chemical drugs cannot achieve therapeutic effect, etc., and achieve the effects of inhibiting proliferation and migration, inhibiting growth and migration, and cheap reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
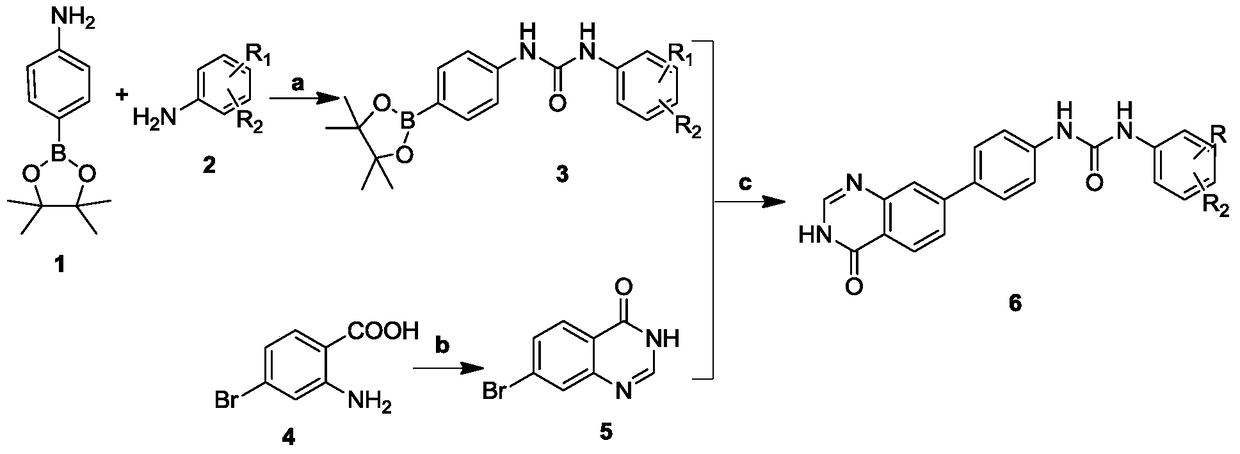
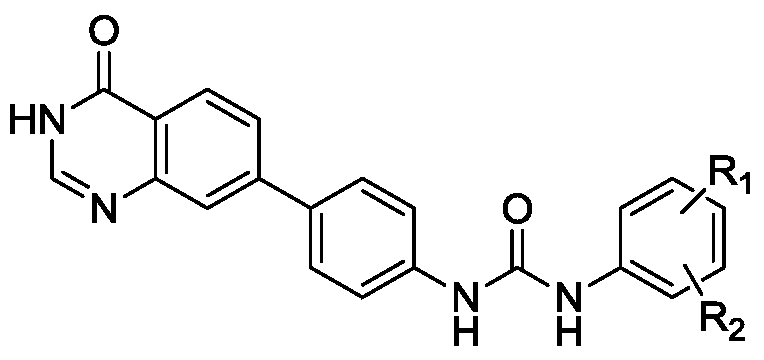
[0033] In the structural formula of the biphenylurea compound containing quinazolinone, R 1 is hydrogen, R 2 for CF 3 , prepared by the following steps (see figure 1 ):
[0034] 1) Preparation of compound 1-(3 trifluoromethyl)phenyl)-3-(4-(4,4 ,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)urea (compound 3)
[0035] Under ice-bath conditions, dissolve 0.80 g (2.74 mmol) of bis(trichloromethyl)carbonate (BTC) with 20 mL of redistilled dichloromethane and stir for 5 min, then slowly add 1.1 g (6.85. Fluoromethylaniline (compound 2) dichloromethane solution, after the dropwise addition, stir for 15min, continue to drop 1.1mL (8.22mmol) triethylamine in dichloromethane solution 10mL into the cloudy solution, and continue stirring for 15min after the dropwise addition , then in the reaction solution, dropwise add 1.50g (6.85mmol) 4-aminophenylboronic acid pinacol ester (compound 1) and 1.1mL (8.22mmol) triethylamine dichloromethane solution 10mL, continue to stir after dropwi...
Embodiment 2
[0046] R in the biphenylurea compound containing quinazolinone 1 , R 2 Both are fluorine.
[0047] Steps 1-2 are the same as Steps 1-2 in Example 1, that is, compound 1-( 3,4-difluoro)phenyl)-3-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)urea ( Compound 3). Simultaneously, 7-bromoquinazolin-4(3H)-one (compound 5) was prepared by cyclization reaction between 2-amino-4-bromobenzoic acid (compound 4) and formamide.
[0048] Step 3: 1-(3,4-Difluoro)phenyl)-3-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl ) phenyl)urea (compound 3) and compound 7-bromoquinazolin-4(3H)-one (compound 5) were prepared by Suzuki coupling reaction to prepare 1-(4-(4-oxo-3,4-di Hydroquinazolin-7-yl)phenyl)-3-(3,4-difluoro)phenyl)urea (Compound 6). The specific operation steps are:
[0049] 1-(3,4-difluoro)phenyl)-3-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzene Base) urea (compound 3) 1.40g (3.74mmol), 7-bromoquinazolin-4 (3H)-one (compound 5) 0.84g (3.74mmol), potassium carb...
Embodiment 3
[0056] R in the biphenylurea compound containing quinazolinone 1 for hydrogen, R 2 for OCF 3 .
[0057] Steps 1-2 are the same as steps 1-2 in Example 1, that is, compound 1-(compound 1-( 4-trifluoromethoxy)phenyl)-3-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)urea (Compound 3). Simultaneously, 7-bromoquinazolin-4(3H)-one (compound 5) was prepared by cyclization reaction between 2-amino-4-bromobenzoic acid (compound 4) and formamide.
[0058] Step 3: 1-(4-Trifluoromethoxy)phenyl)-3-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-2- 1-(4-(4-oxo-3,4- Dihydroquinazolin-7-yl)phenyl)-3-(4-trifluoromethoxy)phenyl)urea (Compound 6). The specific operation steps are:
[0059] 1-(4-trifluoromethoxy)phenyl)-3-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) Phenyl) urea (compound 3) 1.40g (3.32mmol), 7-bromoquinazolin-4 (3H)-one (compound 5) 0.82g (3.32mmol), potassium carbonate 1.37g (9.96mmol), four ( Dissolve 0.38 g (0.33 mmol) of triphenylphosphine)palladium in 90 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com