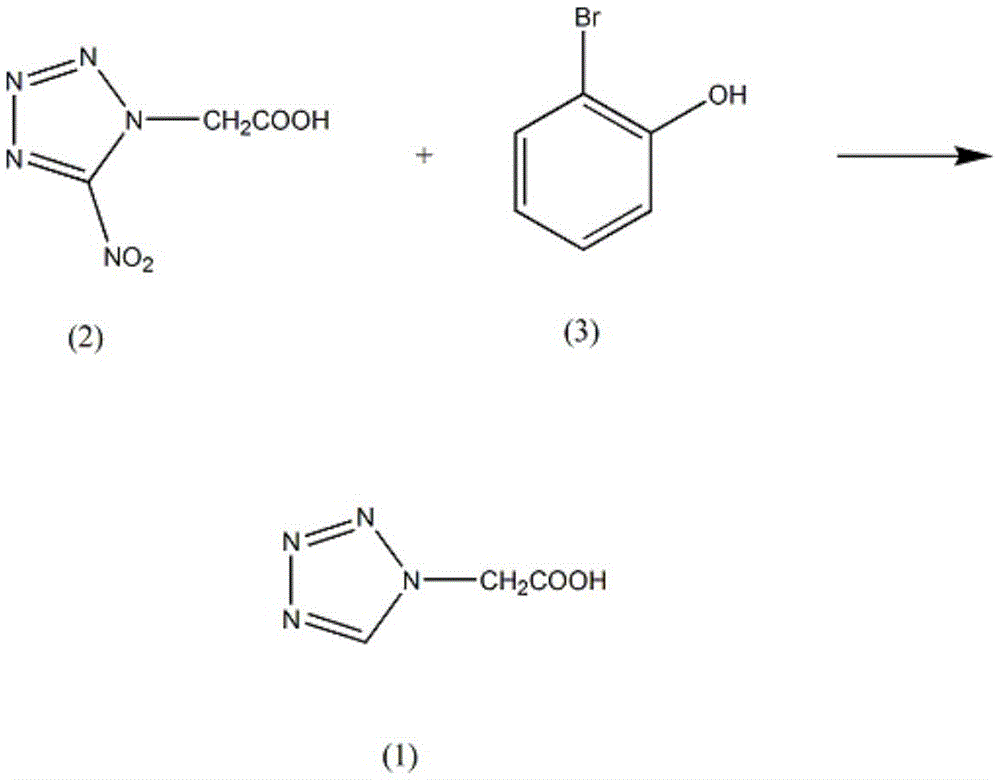
Synthetic method of ceftezol drug intermediate 2-(1H-1-tetrazolyl)acetic acid
A technology of demetazoline cephalosporin and synthetic method, which is applied in the field of synthesis of 2-acetic acid, a drug intermediate of demetazoline cephalosporin, can solve the problems of poor antibacterial effect and poor antibacterial effect of Acinetobacter, and achieve The effect of reducing intermediate links, reducing reaction temperature and reaction time, and increasing reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0011] (i) Add 0.51mol of 2-(5-nitro-1H-1-tetrazolyl)acetic acid (2) into the reaction vessel, the mass fraction is 0.56mol of 65% o-bromophenol solution (3), and the stirring speed is controlled at 130rpm , add cuprous chloride 0.26mol, mass fraction is 15% potassium chloride solution 310ml, control the solution temperature at 10°C, react for 7h, lower the solution temperature to 5°C, add mass fraction of 35% ammonium nitrite solution to adjust pH maintenance At 6, extract 5 times with mass fraction of 50% dimethyl sulfoxide, combine organic layers, wash with mass fraction of 60% cyclohexanol, dehydrate phosphorus pentoxide, evaporate dimethyl sulfoxide under reduced pressure, and add the residue to The mass fraction is 70% N-methylformamide, a solid is precipitated, filtered, and recrystallized in 95% acetamide to obtain 45.04 g of crystalline 2-(1H-1-tetrazolyl)acetic acid, with a yield of 69% .
example 2
[0013] Add 0.51mol of 2-(5-nitro-1H-1-tetrazolyl) acetic acid (2) in the reaction vessel, the mass fraction is 67% o-bromophenol solution (3) 0.58mol, control the stirring speed at 140rpm, add chlorine Cuprous chloride 0.26mol, mass fraction is 17% potassium chloride solution 310ml, control the solution temperature at 12°C, react for 8h, lower the solution temperature to 7°C, add mass fraction of 37% ammonium nitrite solution to adjust the pH to maintain at 6, Extract 6 times with 52% dimethyl sulfoxide in mass fraction, combine the organic layers, wash with 63% cyclohexanol in mass fraction, dehydrate with solid sodium hydroxide, evaporate dimethyl sulfoxide under reduced pressure, and add the residue to 72% N-methylformamide, a solid was precipitated, filtered, and recrystallized in 96% acetamide by mass fraction to obtain 47.65 g of crystalline 2-(1H-1-tetrazolyl)acetic acid with a yield of 73%.
example 3
[0015] Add 0.51mol of 2-(5-nitro-1H-1-tetrazolyl) acetic acid (2) in the reaction vessel, the mass fraction is 0.59mol of 70% o-bromophenol solution (3), control the stirring speed at 150rpm, add chlorine Cuprous chloride 0.26mol, mass fraction is 20% potassium chloride solution 310ml, control the solution temperature at 15°C, react for 9h, lower the solution temperature to 8°C, add mass fraction of 40% ammonium nitrite solution to adjust the pH to maintain at 7, Extract 7 times with 55% dimethyl sulfoxide, combine the organic layers, wash with 65% cyclohexanol, dehydrate phosphorus pentoxide, evaporate dimethyl sulfoxide under reduced pressure, and add the residue to 75% N-methylformamide, a solid was precipitated, filtered, and recrystallized in 98% acetamide to obtain 53.53 g of crystalline 2-(1H-1-tetrazolyl)acetic acid, with a yield of 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com