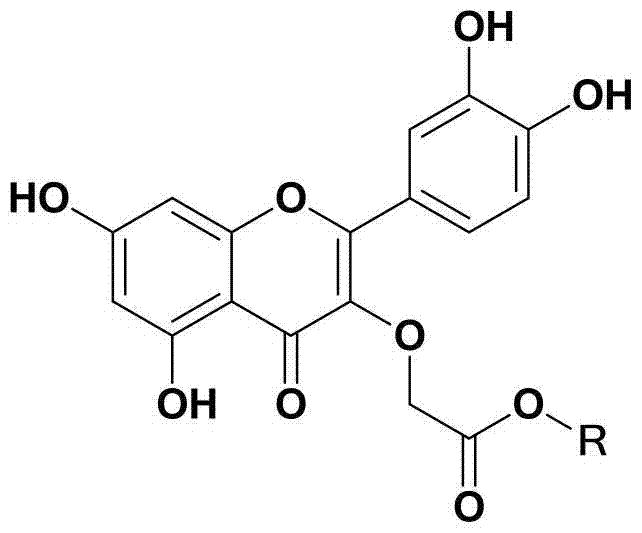
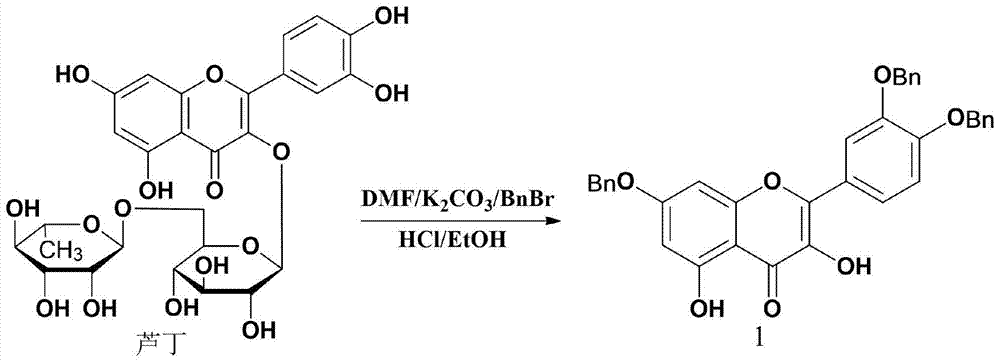
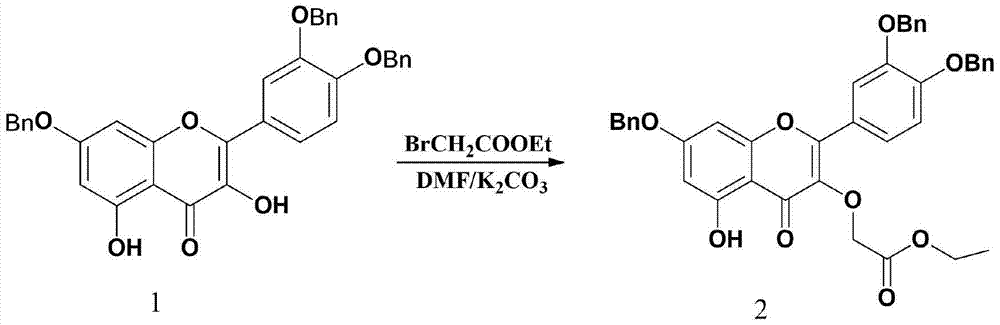
Synthesis of Quercetin‑3‑o‑Acetate and Its Application in Antitumor
A technology of quercetin and its use, which is applied in the chemical field and can solve the problems of unfavorable industrial production and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1 Synthesis of the compound quercetin-3-O-ethyl acetate
[0018] 1 Experimental instruments and materials
[0019] 1.1 Instruments and equipment
[0020] Rotary evaporator: RE-52AA, Shanghai Yarong Biochemical Instrument Factory; Circulating water vacuum pump: SHZ-(Ⅲ) type, Tianjin Huaxin Instrument Factory;
[0021] Constant temperature magnetic stirrer: 85-2 type, Shanghai Sile Instrument Co., Ltd.; infrared spectrometer, iS10FT-IR type, Nicholas, USA; superconducting nuclear magnetic resonance apparatus, 400MHz type, Bruker (DMSO-d 6 or CDCl 3 For solvent, TMS is internal standard); Hydrogenation unit:
[0022] 1.2 Drugs and reagents
[0023] Rutin: purity > 95%, Henan Pingyu Xinxing Biochemical Co., Ltd.; ethyl bromoacetate: purity 98%, Shanghai Jingchun Reagent Co., Ltd.; benzyl bromide: 99%, Shanghai Jingchun Reagent Co., Ltd.; palladium carbon : 10% Pd, Shanghai Jingchun Reagent Co., Ltd.; N,N-dimethylformamide, ethyl acetate, anhydrous potassium car...
Embodiment 2
[0031] Example 2 Synthesis of the compound quercetin-3-O-methyl acetate
[0032] 1.1 Synthesis of 3′,4′,7-O-tribenzylquercetin-3-O-methyl acetate (4)
[0033] Dissolve 2.86g (5mmol) of dry compound 1 in 60ml DMF, add anhydrous K 2 CO 3 900mg (6.5mmol), stirred at room temperature for 30min, then slowly added dropwise a solution of 735mg (5.5mmol) methyl bromoacetate in DMF (20ml), reacted at room temperature for 2h, TLC monitored the completion of the reaction. Adjust the pH to 6-7 with glacial acetic acid, then extract with ethyl acetate / water (100 / 80ml), wash with saturated brine, dry the ester layer with anhydrous sodium sulfate overnight, filter, concentrate under reduced pressure, pass through a short column (acetone / petroleum ether 1:4) to obtain 2.76g of compound 4, yield 85.7%. 1 H NMR (400MHz, CDCl 3 )δ12.49 (s, 1H, 5-OH), 7.89 (d, J = 2.1Hz, 1H), 7.74 (dd, J = 8.6, 2.1Hz, 1H), 7.54–7.31 (m, 15H), 7.05 (d, J=8.7Hz, 1H), 6.47(dd, J=20.1, 2.2Hz, 2H), 5.30(d, J=5....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com