Synthesis method for 2, 5-dimethyl phenylacetic acid
A technology of dimethylphenylacetic acid and a synthetic method, which is applied in the field of compound preparation, can solve problems such as low yield of synthetic products, highly toxic reaction raw materials, etc., and achieve the effects of cheap raw materials, easy large-scale industrial production, and simple method and process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
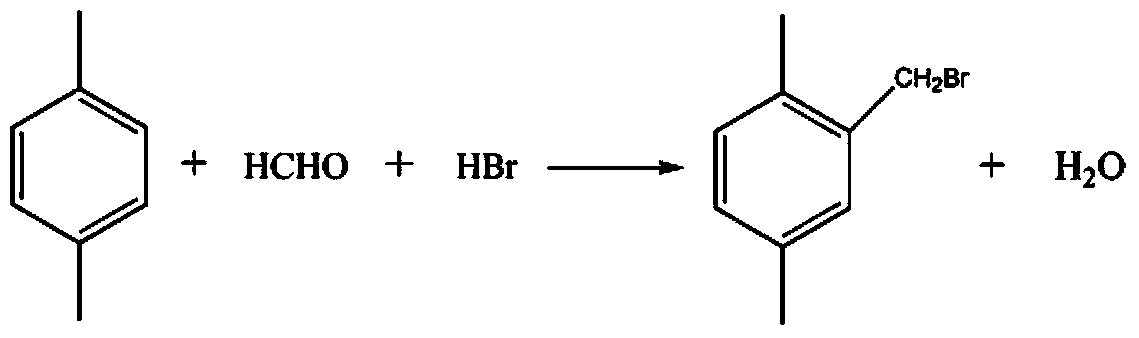
[0034] (1) Take 16g of p-xylene and 4.5g of paraformaldehyde in a 250ml four-neck bottle, add 75ml of glacial acetic acid and stir well. At room temperature, while vigorously stirring at 1000rpm, another 12ml of 47% (wt) hydrogen bromide aqueous solution was added dropwise to the mixture, and the dropwise addition was completed in about 1 hour. Then, the temperature was raised to 60°C, and the reaction was refluxed for 4 hours. After cooling to room temperature, the solution was poured into 300ml of distilled water, allowed to stand for liquid separation, and the organic phase was separated, dried with anhydrous sodium sulfate, and filtered. The filtrate was distilled under reduced pressure (vacuum degree: 0.09 MPa, temperature: 50°C), and after glacial acetic acid and unreacted reactants were evaporated, 12.34 g of 2,5-dimethylbenzyl bromide was obtained with a yield of 62%.
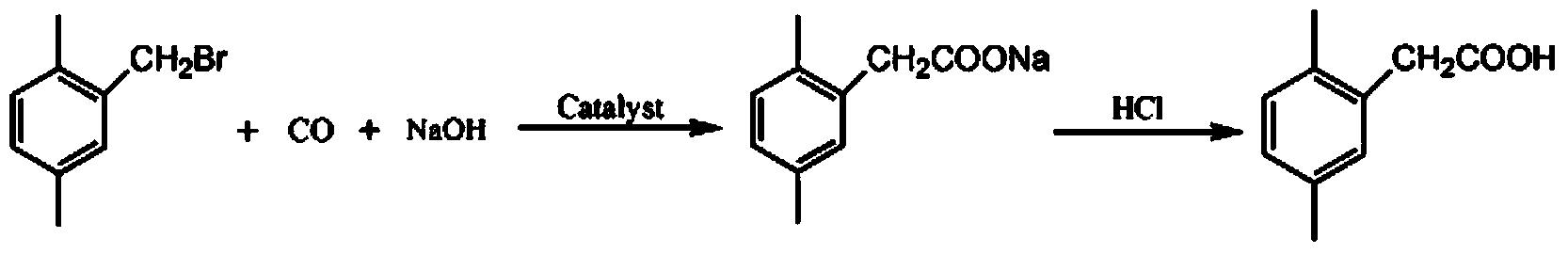
[0035] (2) Add 10.0g 2,5-dimethylbenzyl bromide, 2g sodium hydroxide (2,5-dimethylbenzyl bromide:sod...
Embodiment 2
[0037] (1) Take 16g of p-xylene and 4.5g of paraformaldehyde in a 250ml four-neck bottle, add 75ml of glacial acetic acid and stir well. At room temperature, while vigorously stirring at 1500rpm, another 17ml of 47% (wt) hydrogen bromide aqueous solution was added dropwise to the mixture, and the dropwise addition was completed in about 1 hour. The temperature was raised to 70°C, and the reaction was refluxed for 6 hours. After cooling to room temperature, the solution was poured into 300ml of distilled water, allowed to stand for liquid separation, and the organic phase was separated, dried with anhydrous sodium sulfate, and filtered. The filtrate was distilled under reduced pressure (vacuum degree: 0.08MPa, temperature: 60°C), and after glacial acetic acid and unreacted reactants were distilled off, 21.66 g of 2,5-dimethylbenzyl bromide was obtained with a yield of 74%.
[0038] (2) Add 10.0g 2,5-dimethylbenzyl bromide, 2.4g sodium hydroxide (2,5-dimethylbenzyl bromide:sodiu...
Embodiment 3
[0040] (1) Take 16g of p-xylene and 4.5g of paraformaldehyde in a 250ml four-neck bottle, add 75ml of glacial acetic acid and stir well. At room temperature, while stirring vigorously at 500rpm, another 14ml of 47% (wt) hydrogen bromide aqueous solution was added dropwise to the mixture, and the dropwise addition was completed in about 1 hour. Then, the temperature was raised to 80°C, and the reaction was refluxed for 8 hours. After cooling to room temperature, the solution was poured into 300ml of distilled water, allowed to stand for liquid separation, and the organic phase was separated, dried with anhydrous sodium sulfate, and filtered. The filtrate was distilled under reduced pressure (vacuum degree: 0.09 MPa, temperature: 60°C), and after glacial acetic acid and unreacted reactants were evaporated, 16.13 g of 2,5-dimethylbenzyl bromide was obtained with a yield of 68%.
[0041] (2) Add 10.0g 2,5-dimethylbenzyl bromide, 3g sodium hydroxide (2,5-dimethylbenzyl bromide:sodi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com