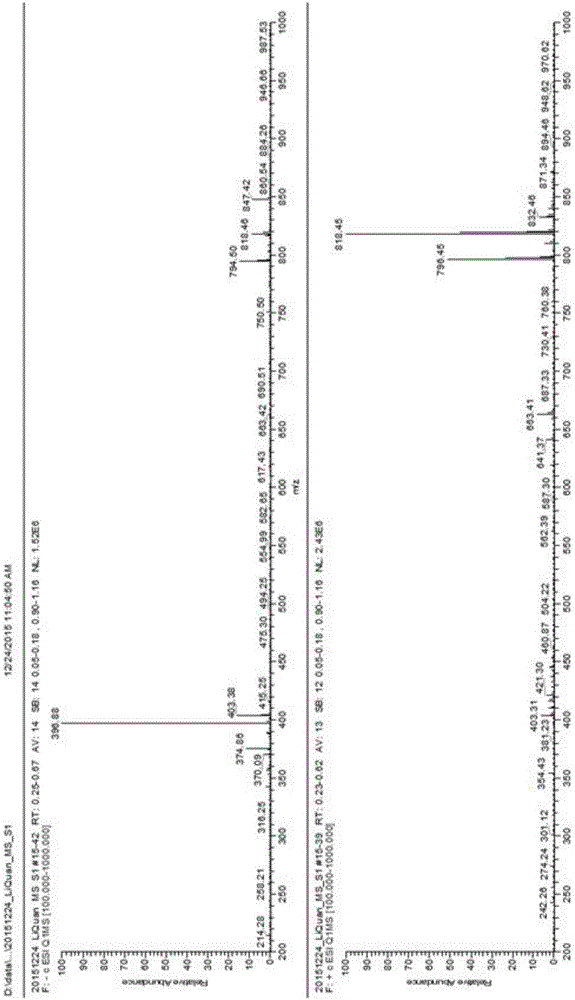
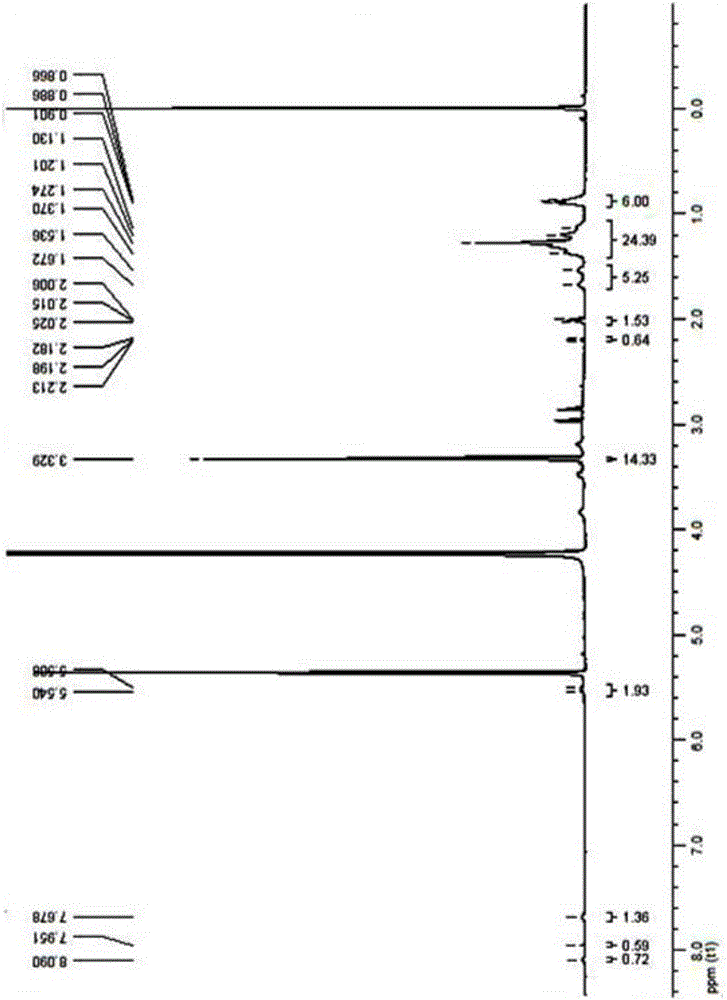
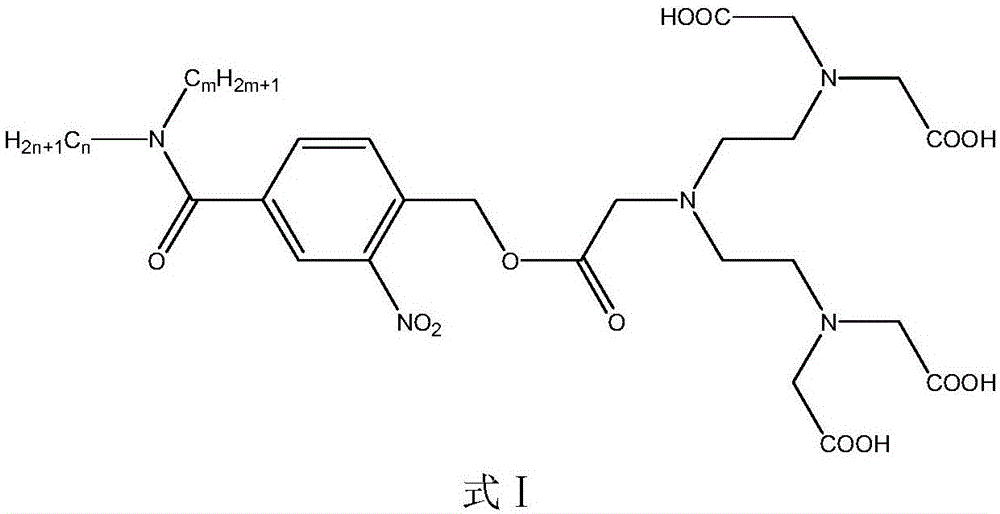
Surfactant containing o-nitrobenzyl ester photodegradation group, and preparation method thereof
A technology of o-nitrobenzyl ester and surfactant, applied in the field of new light-controlled surface active molecules and its preparation, can solve the problems of low efficiency, long reaction time, cumbersome steps, etc., to improve efficiency and shorten complex reaction process , Improve the effect of reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1 The synthesis of photodegradable surfactant (4-diethylenetriaminepentaacetate)-3-nitro-N,N-dioctylbenzamide:
[0042] (1) Synthesis of diethylene triamine pentaacetic anhydride
[0043] Weigh 3.98g (0.01mol) of diethylenetriaminepentaacetic acid and a 100mL single-necked flask, add 4mL of acetic anhydride and 5mL of pyridine in sequence, raise the temperature to 65°C under nitrogen protection, and react for 24h. Suction filtration after the reaction was completed to obtain a white solid powder. Melting point: 178°C-184°C
[0044] (2) Synthesis of intermediates of N,N-dioctylbenzamide brominated derivatives
[0045]Take 1g (0.004mol) of 4-bromomethyl-3-nitrobenzoic acid in a 100mL three-necked flask, add 10mL of thionyl chloride dropwise under magnetic stirring, and reflux for 3 hours. sulfone. Add 0.96g (0.004mol) di-n-octylamine dropwise to the three-necked flask. After the dropwise addition, add 0.5g (0.004mol) potassium carbonate, react at 40°C for 1...
Embodiment 2
[0052] Example 2 Synthesis of photodegradable surfactant (4-diethylenetriaminepentaacetate)-3-nitro-N,N-didecylbenzamide:
[0053] (1) Synthesis of diethylene triamine pentaacetic anhydride
[0054] Same as Example 1
[0055] (2) Synthesis of N,N-Didecylbenzamide Brominated Derivative Intermediates
[0056] Take 1g (0.004mol) of 4-bromomethyl-3-nitrobenzoic acid and a 100mL three-necked flask, add 10mL of thionyl chloride dropwise under magnetic stirring, and reflux for 4 hours. sulfone. Add 1.19g (0.004mol) di-n-decylamine dropwise to the three-necked flask. After the dropwise addition, add 0.5g (0.004mol) potassium carbonate, react at 50°C for 15h, stop the reaction, add an appropriate amount of deionized water, separate the liquid, and the organic layer Dry it with anhydrous sodium sulfate, and use a 40:1 volume ratio mixture of dichloromethane and methanol as the eluent for column separation to obtain a pale yellow oily liquid. Yield 62.1%.
[0057] (3) Synthesis of (...
Embodiment 3
[0059] Example 3 Synthesis of photodegradable surface active molecule (4-diethylenetriaminepentaacetate)-3-nitro-N,N-behenylbenzamide:
[0060] (1) Synthesis of diethylene triamine pentaacetic anhydride
[0061] Same as Example 1
[0062] (2) Synthesis of intermediates of brominated derivatives
[0063] Take 1g (0.004mol) of 4-bromomethyl-3-nitrobenzoic acid and a 100mL three-necked flask, add 10mL of thionyl chloride dropwise under magnetic stirring, and reflux for 3 hours. sulfone. Add 1.47g (0.004mol) didodecylamine dropwise to the three-necked flask. After the dropwise addition, add 0.5g (0.004mol) potassium carbonate, react at 40°C for 12 hours, stop the reaction, add an appropriate amount of deionized water, separate the liquid, and the organic layer Dry it with anhydrous sodium sulfate, and use a 40:1 volume ratio mixture of dichloromethane and methanol as the eluent for column separation to obtain a pale yellow oily liquid. Yield 46.5%.
[0064] (3) Synthesis of (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com