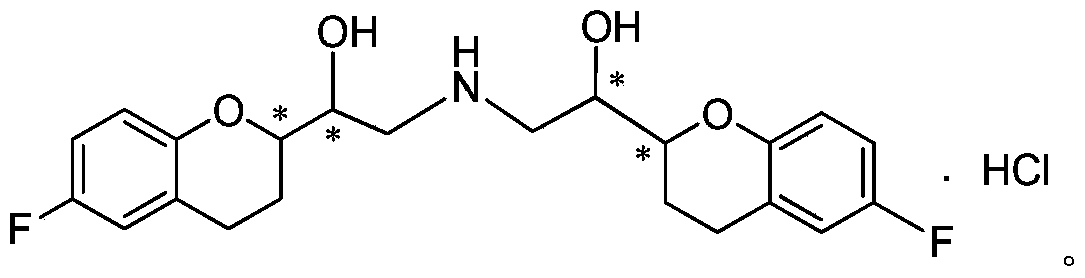
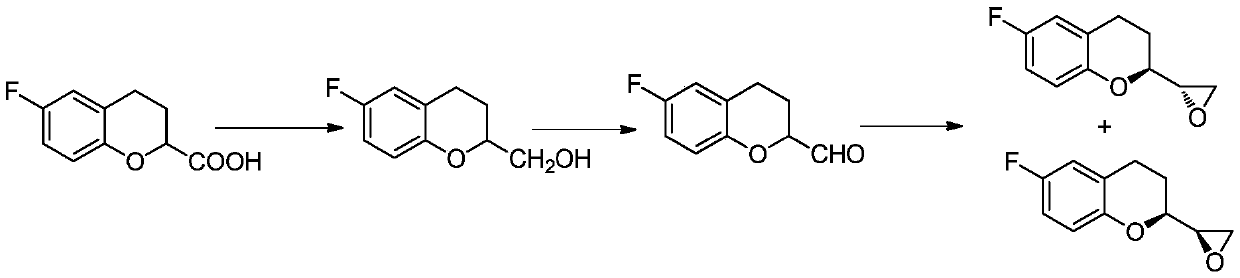
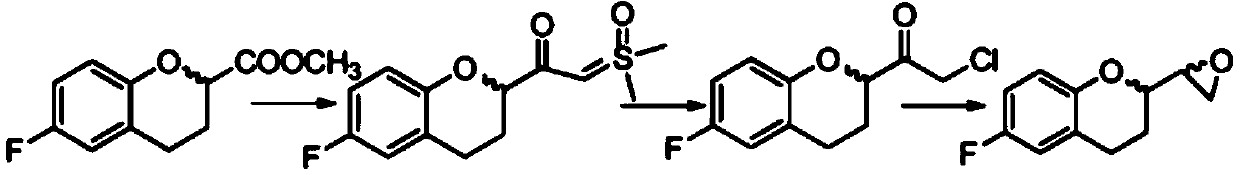
Method for preparing nebivolol hydrochloride epoxy intermediate 6-fluoro-2-epoxy ethyl chroman
A technology of nebivolol hydrochloride epoxy and epoxyethyl chroman, applied in the field of preparation of nebivolol hydrochloride intermediate 6-fluoro-2-oxiranyl chroman, which can solve the active nature of nitrogen salts and potential safety hazards , not suitable for long-term storage and other problems, to achieve the effect of simple operation, high product purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) Add 32g of potassium dihydrogen phosphate and 2kg of water into a 3L reaction flask and stir to dissolve, adjust the pH value to 7.0-7.4 with 2mol / L NaOH solution, then add 40g of zinc chloride, 40g of ammonium formate, and 200g of aldose reductase in sequence , NADPH 20g, (S)-2-chloro-1-(6-fluorochroman-2-yl)ethanone 100g, use 2mol / L sodium hydroxide solution to adjust the pH value to 7.0-7.4, at 55℃±2℃ React for about 12 hours, filter with suction, wash the filter cake with 200ml ethyl acetate, separate the layers, take the water phase and wash it with 200ml ethyl acetate × 3, combine the organic phases and evaporate to dryness under reduced pressure to obtain (S,S)-2-chloro -1-(6-fluorochroman-2-yl)ethanol 95.6g, yield 94.8%, ee%>99.5%;
[0042] (2) Take 90 g of (S, S)-2-chloro-1-(6-fluorochroman-2-yl) ethanol obtained in step (1), 220 g of ethanol, and 16.2 g of sodium hydroxide, and add them into a dry reaction bottle, Raise the temperature to reflux for 4 hou...
Embodiment 2
[0044](1) Add 53g of sodium dihydrogen phosphate and 4kg of water into a 3L reaction flask and stir to dissolve, adjust the pH value to 6.8-7.8 with 2mol / L NaOH solution, then add 78g of zinc chloride, 80g of ammonium formate, and 400g of aldose reductase in sequence , NADPH42g, (S)-2-chloro-1-(6-fluorochroman-2-yl)ethanone 100g, use 2mol / L sodium hydroxide solution to adjust the pH value to 6.8-7.8, at 55℃±2℃ React for about 12 hours, filter with suction, wash the filter cake with 400ml of ethyl acetate, separate layers, take the water phase and wash with 400ml of ethyl acetate × 3, combine the organic phases and evaporate to dryness under reduced pressure to obtain (S,S)-2-chloro -1-(6-fluorochroman-2-yl)ethanol 93.8g, yield 93%, ee%>99.5%;
[0045] (2) Take 90 g of (S, S)-2-chloro-1-(6-fluorochroman-2-yl)ethanol obtained in step (1), 230 g of methanol, and 22.7 g of potassium hydroxide, and add them into a dry reaction flask, Raise the temperature to reflux for 4 hours, fi...
Embodiment 3
[0047] (1) Add 60g of potassium dihydrogen phosphate and 4kg of water into a 3L reaction flask and stir to dissolve, adjust the pH value to 6.8-7.8 with 2mol / L NaOH solution, then add 78g of zinc chloride, 80g of ammonium formate, and 400g of aldose reductase in sequence , NADPH42g, (R)-2-chloro-1-(6-fluorochroman-2-yl)ethanone 100g, use 2mol / L sodium hydroxide solution to adjust the pH value to 6.0-8.0, at 55℃±2℃ React for 12-14 hours, filter with suction, wash the filter cake with 400ml of ethyl acetate, separate layers, take the water phase and wash with 400ml of ethyl acetate×3, combine the organic phases and evaporate to dryness under reduced pressure to obtain (R,R)-2- Chloro-1-(6-fluorochroman-2-yl)ethanol 92g, yield 91.2%, ee%>99.5%;
[0048] (2) Take 90g of (R,R)-2-chloro-1-(6-fluorochroman-2-yl)ethanol obtained in step (1), 240g of isopropanol, and 22.7g of potassium hydroxide, and add to dry reaction bottle, heated to reflux for 4 hours, filtered, evaporated the mo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com