A kind of swine fever antibody detection system and preparation method thereof
A swine fever antibody and swine fever virus technology, applied in the fields of molecular biology and livestock disease diagnosis, can solve problems such as false negatives, and achieve the effect of improving sensitivity and high capture efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The preparation of embodiment 1 swine fever E2 protein
[0053] According to the gene sequence (see sequence table SEQ ID No.1) of the E2 protein in the CSFV C strain (accession number is AF531433) reported in NCBI (http: / / www.ncbi.nlm.nih.gov) to prepare CSF Viral E2 protein.
[0054] 1.1 Construction of pFastBac HBM-E2
[0055] Design a pair of primers to amplify E2 protein by PCR:
[0056] CE2-F: 5'-CGGCTAGCCTGCAAGGAAGATTAC-3'
[0057] CE2-R: 5'-TCTTTCATCTGATGCATGCACCT-3'
[0058] PCR reaction conditions: pre-denaturation at 98°C for 2min, denaturation at 98°C for 10s, annealing at 55°C for 15s, extension at 72°C for 1min, 30 cycles, and extension at 72°C for 7min. Use 1% agarose gel to electrophoresis the amplified product. After the electrophoresis product is recovered using a DNA gel recovery kit, the target gene fragment is connected to the pFastBac HBM vector. The connection conditions are: 1 μl of carrier, 1 μl of carrier buffer and 4 μl of gel recovery pro...
Embodiment 2
[0066] The preparation of embodiment 2 swine fever Erns protein
[0067] According to the gene sequence (see sequence table SEQ ID No.3) of Erns protein in the CSFV C strain (accession number is AF531433) reported in NCBI (http: / / www.ncbi.nlm.nih.gov) to prepare CSF Viral Erns protein.
[0068] 2.1 Construction of pFastBac HBM-Erns
[0069] The designed primer sequences are as follows
[0070] CErns-F: 5'-GAAAATATAACTCAATGGAACCTGA-3'
[0071] CErns-R: 5'-GGCATAAGCGCCAAACCAGGT-3'
[0072] Refer to Example 1.1 to construct pFastBac HBM-E2.
[0073] 2.2 Construction of Bacmid-Erns, acquisition of Erns protein and its purification and quantification
[0074] Referring to Example 1.2-1.4, Bacmid-E2 was sequentially constructed, recombinant E2 protein was obtained, and the recombinant E2 protein was purified and quantified. The protein concentration was 2.1 mg / ml.
Embodiment 3
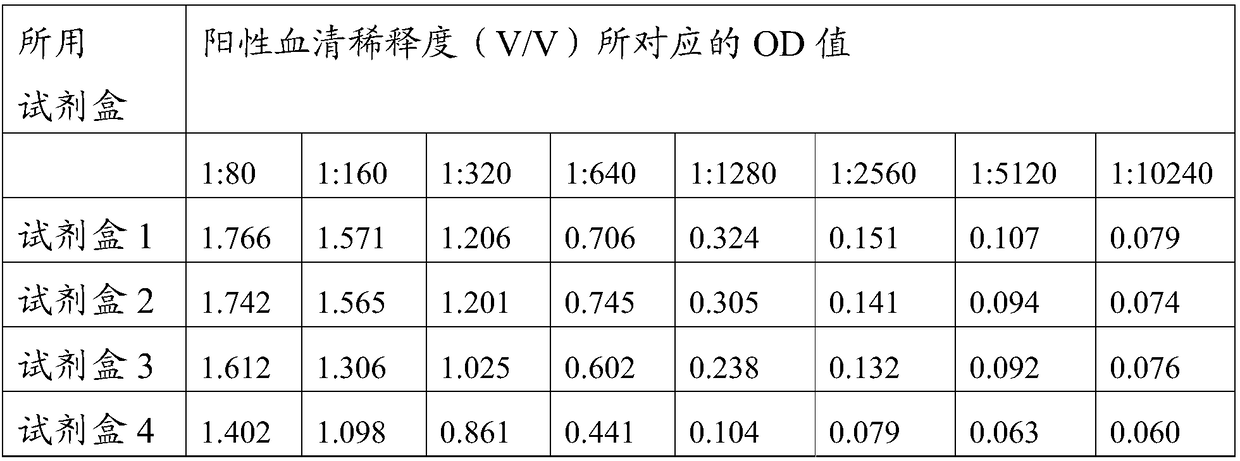
[0075] The preparation of embodiment 3 swine fever antibody ELISA kit
[0076] 3.1 ELISA plate preparation
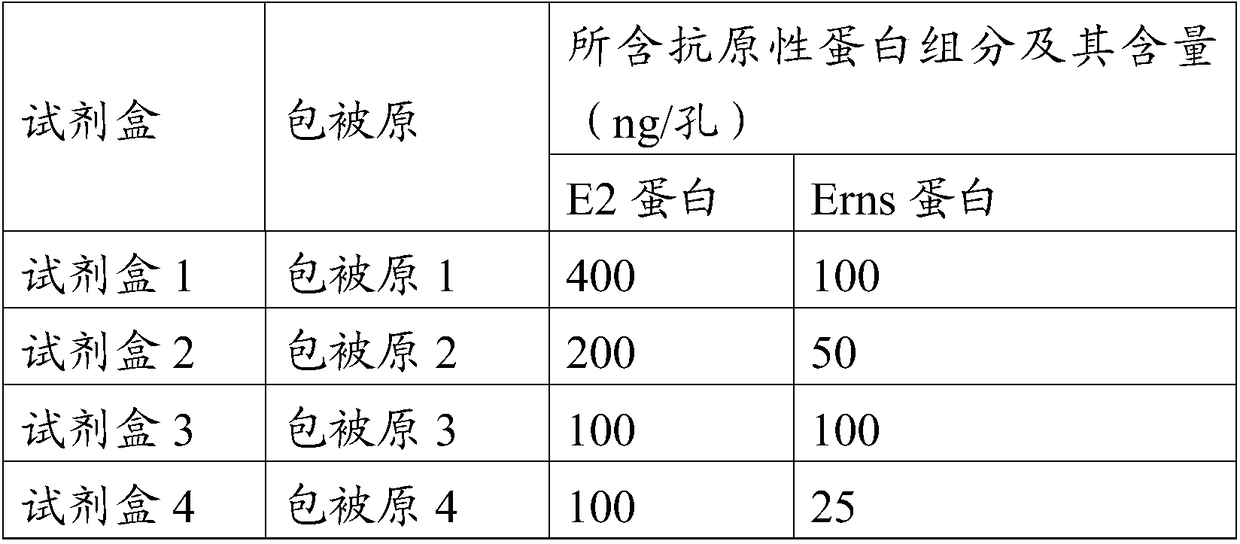
[0077] (1) Enzyme plate coating antigen: the swine fever virus E2 and Erns protein prepared in Example 1-2 were coated with the components corresponding to the original numbering of each coating as shown in Table 1 with carbonate buffer solution of pH 9.6 Prepare.
[0078] Table 1 Coating raw components and their contents corresponding to each kit
[0079]
[0080] (2) Blocking: the blocking solution is 10% skimmed milk diluted in PBST, 100 μl per well, and blocked at 37° C. for 1 h. After blocking, wash 3 times with PBST.
[0081] 3.2 Preparation of reagents
[0082] Diluent: PBS solution containing 0.05% (V / V) Tween-20 and 10% (V / V) skimmed milk;
[0083] Washing solution: PBS solution containing 0.05% (V / V) Tween-20;
[0084] Chromogenic solution A: add 10ml absolute ethanol to 20mg TMB, then use ddH 2 O is fixed to 100ml;
[0085] Chromogenic solution B: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com