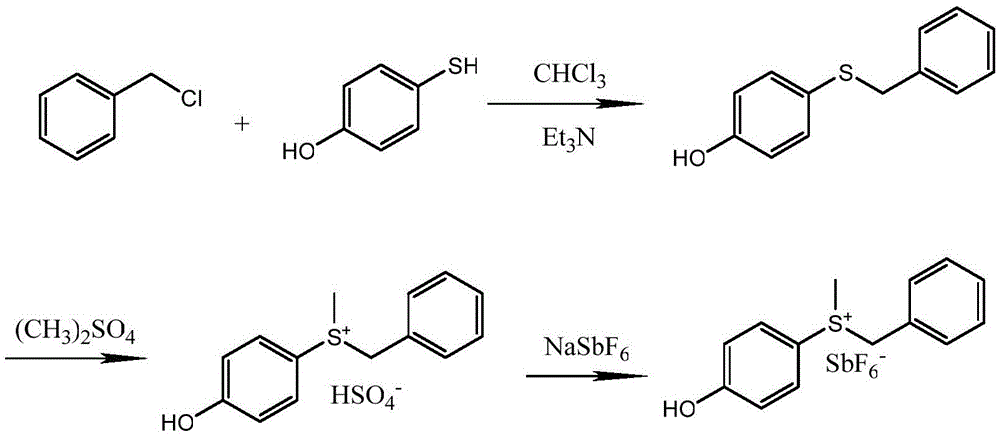
Method for synthesizing (4-hydroxyphenyl) methyl benzyl sulfonium hexafluoroantimonate
A technology of methylbenzylsulfonium hexafluoroantimonate and methylbenzylsulfonium, which is applied in the field of organic chemical industry synthesis, can solve the problem of difficult separation and high purity of 4-hydroxyanisole, 4-hydroxyanisole and 4-hydroxyanisole. The problems such as the difficulty of obtaining the raw materials of anisole sulfide can achieve the effects of simple process, high purity and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1, the preparation of 4-(benzylthio)phenol:
[0028] Add 12.6g (0.1mol) of 4-mercaptophenol and 12.12g (0.12mol) of triethylamine into a three-necked reaction flask with a thermometer and an electric stirrer, and dropwise add 12.6 (0.1mol) of benzyl chloride between 10°C 100ml of chloroform solution, the dropwise addition time is 2h. Then warm up to reflux for 6h. After the reaction is complete, pour it into 150ml of water, separate several layers, wash with 3×100ml of water, dry over anhydrous sodium sulfate, concentrate to obtain a yellow solid 4-(benzylthio)phenol, and then recrystallize with anhydrous methanol to obtain a yellow Solid 20.7g yield 96%. 1 HNMR (400MHz, CDCl 3 )δ7.20-7.05(m,7H),6.66-6.58(m,2H),5.34(s,1H),3.89(s,2H);
[0029] 2. Preparation of (4-hydroxyphenyl)methylbenzylsulfonium sulfate:
[0030] Add 21.6g (0.1mol) of 4-(benzylthio)phenol into a three-necked reaction flask with a thermometer and an electric stirrer, add 150ml of toluene, and add...
Embodiment 2
[0034] 1, the preparation of 4-(benzylthio)phenol:
[0035] Add 12.6g (0.1mol) of 4-mercaptophenol and 16.56g (0.12mol) of potassium carbonate into a three-necked reaction flask with a thermometer and an electric stirrer, and add 100ml of benzyl bromide 17.0 (0.1mol) dropwise between 10°C Toluene solution, the dropwise addition time is 2h. Then warm up to reflux for 6h. After the reaction is complete, pour it into 150ml of water, separate several layers, wash with 3×100ml of water, dry over anhydrous sodium sulfate, concentrate to obtain a yellow solid 4-(benzylthio)phenol, and then recrystallize with anhydrous methanol to obtain a yellow Solid 20.3g yield 94%.
[0036] 2. Preparation of (4-hydroxyphenyl)methylbenzylsulfonium iodide:
[0037] Weigh 21.6g (0.1mol) of 4-(benzylthio)phenol into a three-necked reaction flask with a thermometer and an electric stirrer, add 150ml of toluene, and drop 50ml of 20.02g (0.12mol) of potassium iodide below 15°C Toluene solution was ad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com