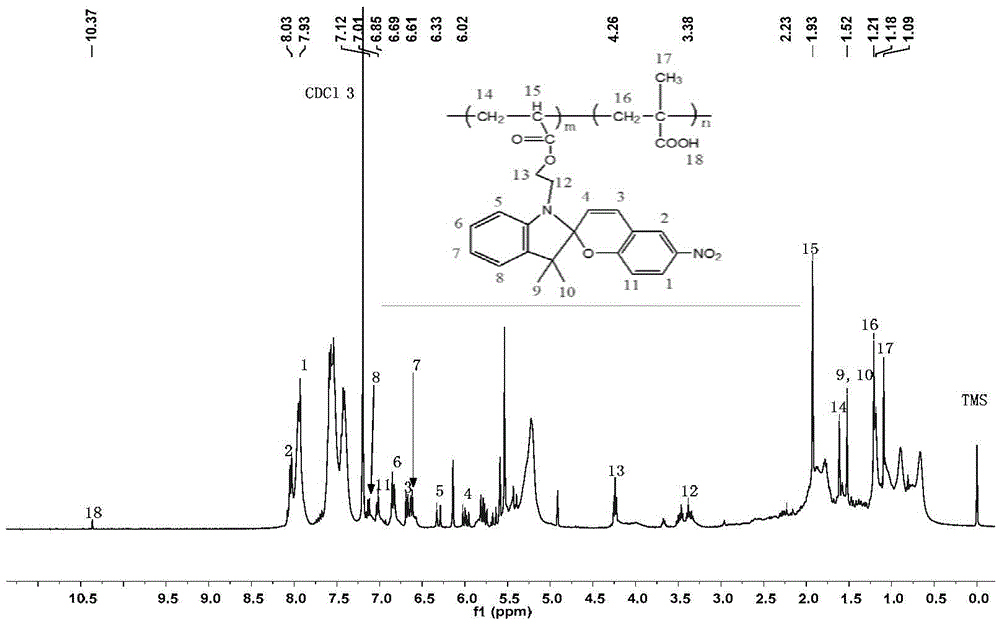
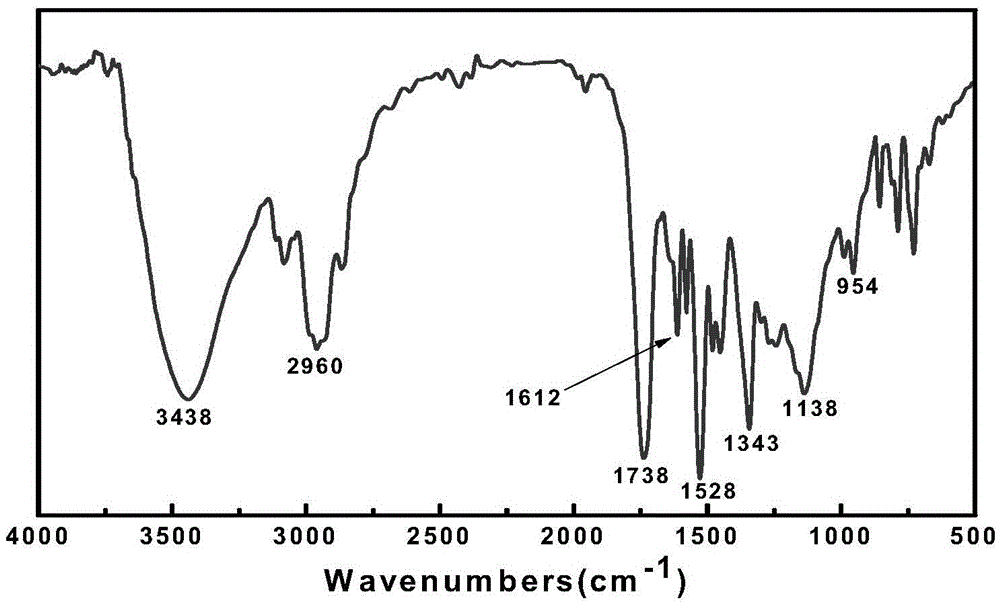
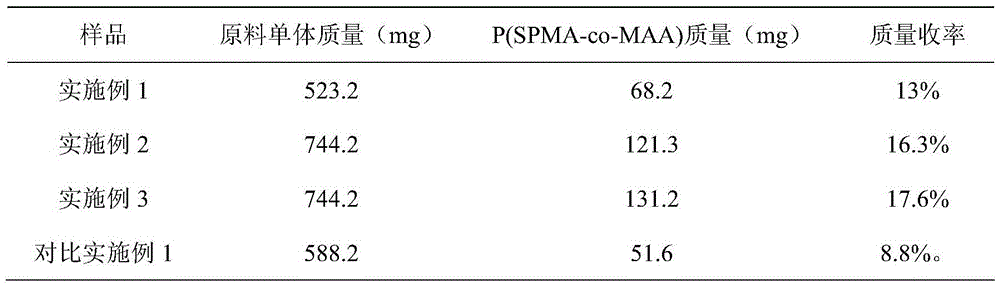
Preparation method for spiropyran-based random copolymer P(SPMA-co-MAA)
A random copolymer, spiropyran-based technology, applied in the field of functional polymer preparation, can solve the problems of polymer loss, complex process, poisoning catalyst, etc., and achieve the effects of fast speed, fast reaction speed and high decomposition efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A stir bar, SPMA (81.2 mg, 0.2 mmol), NBM (442 mg, 2.0 mmol), cuprous bromide (8.7 mg, 0.06 mmol), tetrahydrofuran (3 mL), and ethyl 2-bromoisobutyrate ( 4.4μL, 0.03mmol), after three cycles of freezing-vacuumizing-nitrogen filling to remove the oxygen in the reaction tube and the solvent to prevent catalyst failure; inject N,N,N′,N″,N″-penta with a micro-syringe Diethylenetriamine (84.6 μL, 0.4 mmol), and then the reaction tube was placed in an oil bath at a preset temperature (65° C.) to react for 24 h. After the reaction time is up, expose the reaction product to the air to terminate the reaction, then dissolve the reaction product with tetrahydrofuran and disperse it ultrasonically for 10 minutes, irradiate with ultraviolet light with a wavelength of 365nm for 30 minutes, and pass through a neutral alumina column to remove the residual copper-based catalyst . After the obtained filtrate was concentrated, it was precipitated in 150 mL of n-hexane at 0° C., and the p...
Embodiment 2
[0031] A stir bar, SPMA (81.2 mg, 0.2 mmol), NBM (663 mg, 3.0 mmol), cuprous bromide (8.7 mg, 0.06 mmol), tetrahydrofuran (3 mL), and ethyl 2-bromoisobutyrate ( 4.4μL, 0.03mmol), after three cycles of freezing-vacuumizing-nitrogen filling to remove the oxygen in the reaction tube and the solvent to prevent catalyst failure; inject N,N,N′,N″,N″-penta with a micro-syringe Diethylenetriamine (84.6 μL, 0.4 mmol), and then the reaction tube was placed in an oil bath at a preset temperature (65° C.) to react for 36 h. After the reaction time is up, expose the reaction product to the air to terminate the reaction, then dissolve the obtained reaction product with tetrahydrofuran and disperse it ultrasonically for 20 minutes. . After the obtained filtrate was concentrated, it was precipitated in 150 mL of n-hexane at 0° C., and the product was washed with n-hexane for 3 times, and the obtained product was vacuum-dried. The weight of the product was weighed to be 121.3 mg.
Embodiment 3
[0033]A stir bar, SPMA (81.2 mg, 0.2 mmol), NBM (663 mg, 3.0 mmol), cuprous bromide (8.7 mg, 0.06 mmol), tetrahydrofuran (3 mL), and ethyl 2-bromoisobutyrate ( 4.4μL, 0.03mmol), after three cycles of freezing-vacuumizing-nitrogen filling to remove the oxygen in the reaction tube and the solvent to prevent catalyst failure; inject N,N,N′,N″,N″-penta with a micro-syringe Diethylenetriamine (84.6 μL, 0.4 mmol), and then the reaction tube was placed in an oil bath at a preset temperature (80° C.) to react for 36 h. After the reaction time is up, expose the reaction product to the air to terminate the reaction, then dissolve the obtained reaction product with tetrahydrofuran and disperse it ultrasonically for 30 minutes. . After the obtained filtrate was concentrated, it was precipitated in 150 mL of n-hexane at 0°C, and the product was washed with n-hexane for 3 times, and the obtained product was vacuum-dried, and the weight of the product was weighed to be 131.2 mg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com