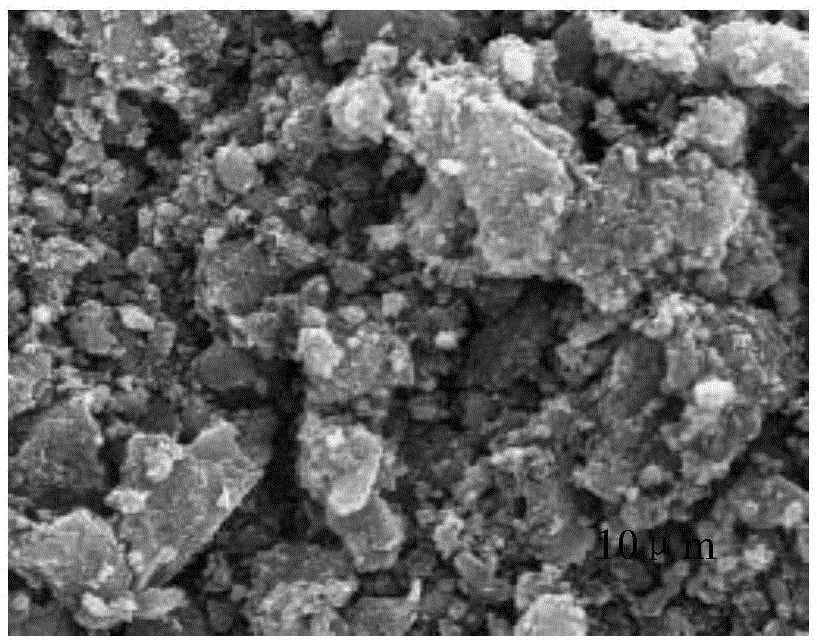
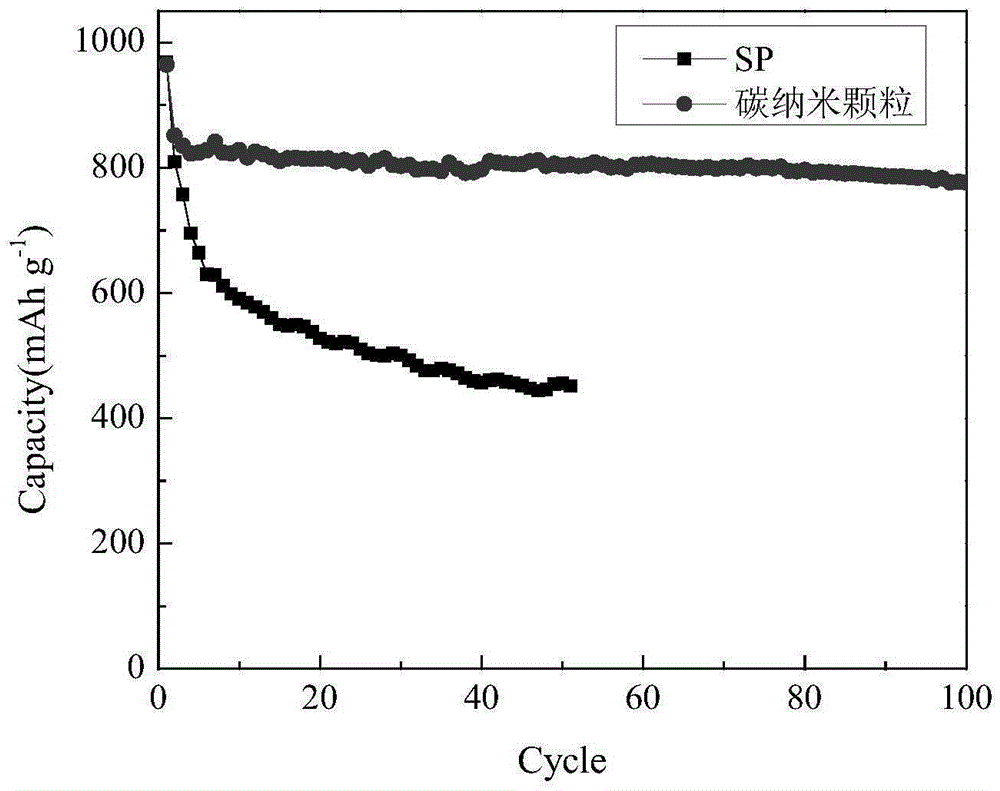
Sulfur-carbon compound and preparation method therefor, and electrode material and lithium-sulfur battery containing sulfur-carbon compound
A technology of sulfur-carbon composites and lithium-sulfur batteries, applied in the field of lithium-ion batteries, can solve problems such as hindering practical applications, changes in shape and structure, and reducing ion conductivity, so as to avoid electrochemical intermediate reactions and the preparation method is simple and easy Line, prevent the effect of the shuttle effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] When the above-mentioned sulfur-carbon composite is prepared, the key lies in the carbon nanoparticles, and its preparation method includes the following steps:
[0048] 11) Provide an aqueous suspension of nano-scale Na-X zeolite, drop strong acid thereinto until the aqueous suspension becomes clear, and obtain an aqueous solution;
[0049] 12) after the ethanol solution of described aqueous solution and phenolic resin is mixed, then react with strong ammonia water, evaporate to dryness solvent (water and ethanol) after reaction is complete, obtain the mixture of carbon nanometer precursor and templating agent;
[0050] 13) carbonizing the mixture of the carbon nano precursor and the template in a protective atmosphere to obtain primary carbon nanoparticles,
[0051] 14) Pickling the primary carbon nanoparticles until the content of the silica template is 1 wt% to 5 wt%, to obtain the carbon nanoparticles.
[0052] In the preparation of carbon nanoparticles, nano-scal...
Embodiment 1
[0063] A kind of sulfur-carbon compound, its preparation method comprises the steps:
[0064]In the first step, take 4 g of the nano-Na-X zeolite with a size of 40 nm prepared by the above method and disperse it in 20 mL of deionized water, add 6 M concentrated hydrochloric acid dropwise, and stir while adding until a clear and translucent aqueous solution is obtained;
[0065] The second step is to prepare carbon nanoparticles: mix the above aqueous solution with 70ml of ethanol solution containing 3wt% phenolic resin, and stir at 50°C to obtain a mixed solution; mix 30mL of concentrated ammonia water with 100mL of ethanol, add the above mixed solution, and stir at 50°C 3h, followed by stirring and evaporating to dryness at 60° C., removing the solvent (ethanol and water) to obtain a mixture of carbon nano precursor and template;
[0066] The third step is to carbonize the mixture of carbon nano-precursor and template agent in a protective atmosphere above 850°C, and then rem...
Embodiment 2
[0070] A kind of sulfur-carbon compound, its preparation method comprises the steps:
[0071] In the first step, take 4 g of the nano-Na-X zeolite with a size of 20 nm prepared by the above method and disperse it in 20 mL of deionized water, add 8 M concentrated hydrochloric acid dropwise, and stir while adding until a clear and translucent aqueous solution is obtained;
[0072] The second step is to prepare carbon nanoparticles: mix the above aqueous solution with 80ml ethanol solution containing 3wt% phenolic resin, and stir at 50°C to obtain a mixed solution; mix 30mL concentrated ammonia water with 100mL ethanol, add the above mixed solution, and stir at 50°C 3h, followed by stirring and evaporating to dryness at 60° C., removing the solvent (ethanol and water) to obtain a mixture of carbon nano precursor and template;
[0073] The third step is to carbonize the mixture of carbon nano-precursor and template agent in a protective atmosphere above 850°C, and then remove the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com