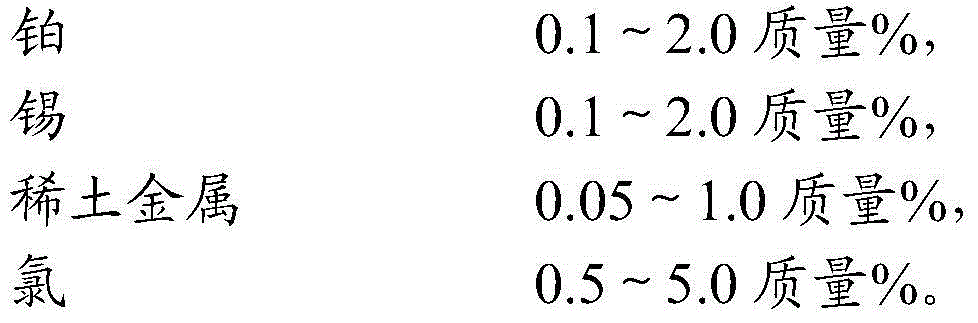Preparation method of polymetallic reforming catalyst
A reforming catalyst, catalyst technology, applied in chemical instruments and methods, physical/chemical process catalysts, naphtha catalytic reforming, etc., can solve the problems of catalyst activity decline, gasoline yield decline, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0038] (1) Preparation of tin-containing γ-Al with uniform distribution of tin 2 o 3 small ball.
[0039] By the method of CN1150169A example 1, 100 grams of SB aluminum hydroxide powder (Germany, produced by Condea company) and an appropriate amount of deionized water are stirred and slurried, and the liquid / solid mass ratio is 2.0. Add 7.5 ml of dilute nitric acid with a volume ratio of 1:1, 30 g of urea and a predetermined amount of SnCl 2 hydrochloric acid solution, so that the Sn content in the solution is 0.30% by mass relative to the dry base alumina, stir for 1 hour, add 30 grams of kerosene and 3 grams of fatty alcohol polyoxyethylene ether and stir for 1 hour, drop balls in the oil ammonia column to form. The wet bulb is solidified in ammonia water for 1 hour, then filtered, rinsed with deionized water for 2 to 3 times, dried at 60°C for 6 hours, dried at 120°C for 10 hours, and calcined at 600°C for 4 hours to obtain Sn-containing γ-Al 2 o 3 A small ball with a ...
example 2
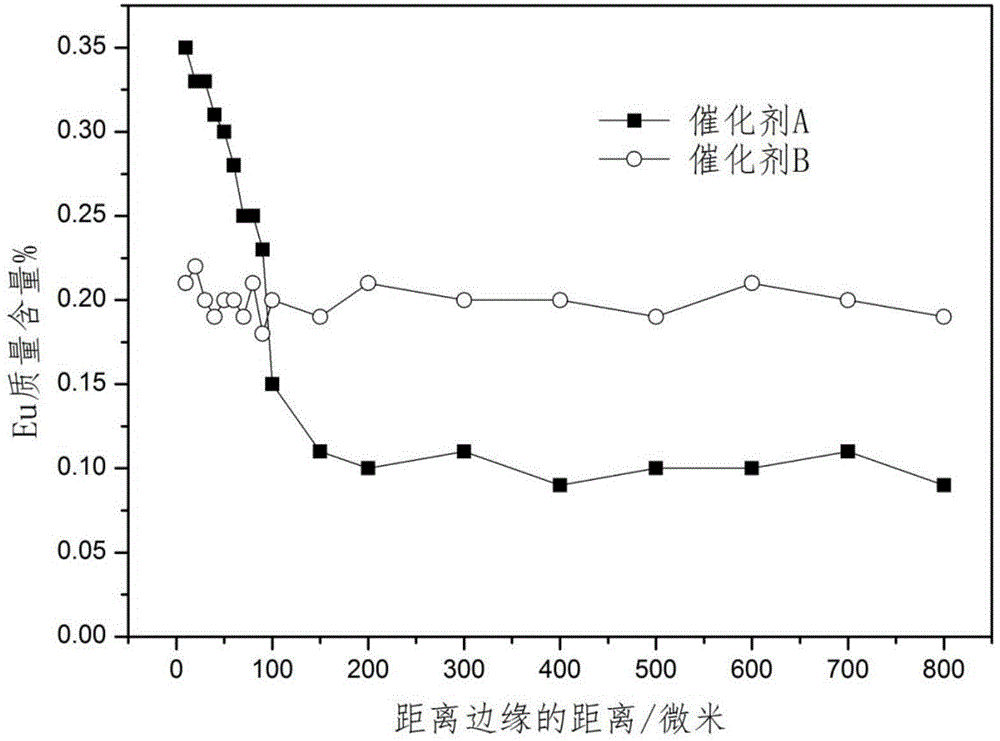
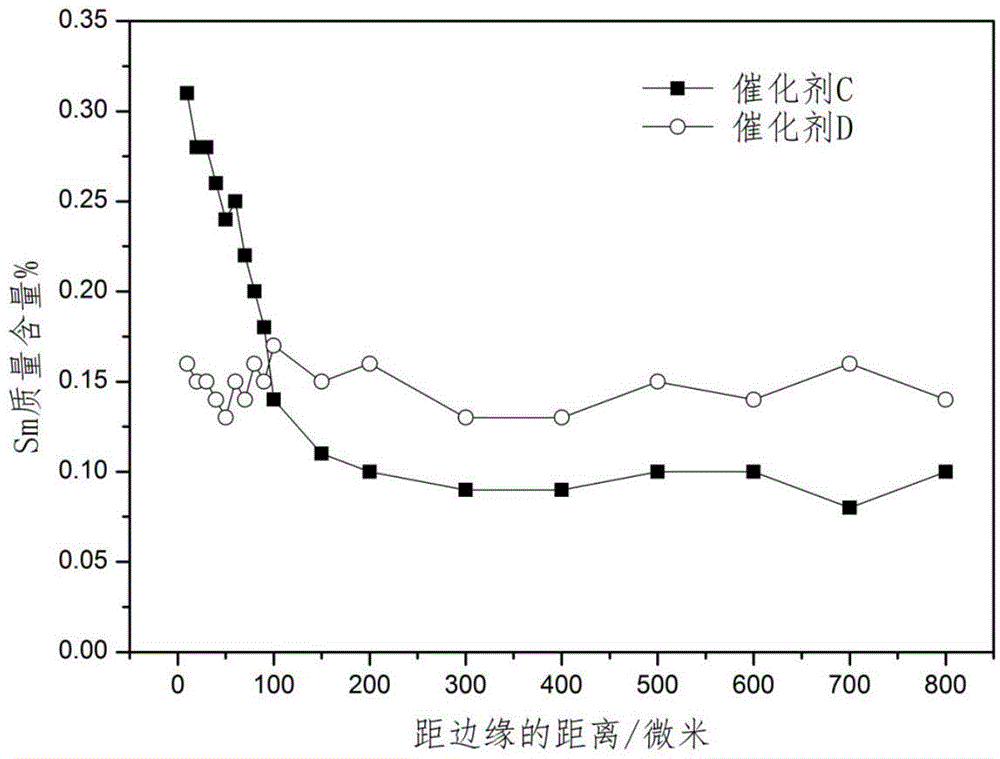
[0045] Prepare catalyst by the method for example 1, difference is (2) step replaces europium nitrate with samarium nitrate, obtains the catalyst precursor that platinum, tin and samarium are uniformly distributed in alumina carrier, wherein contains platinum 0.29 quality %, tin 0.30 quality %, samarium 0.1 mass %, after roasting, press (3) step method and SmCl 3 ·6H 2 O powder carries out solid phase contact at 150 ℃, and the contact time is 10 hours, and the active component content of the prepared catalyst C is: Pt0.29 mass %, Sn0.30 mass %, Sm0.15 mass %, Cl1.10 mass % %, wherein the average content of Sm in the outer shell area is 2.8 times that of the central area, and the distribution of Sm content on the radius of the catalyst pellet section is shown in figure 1 .
example 3~6
[0051] The following examples evaluate the reforming reaction performance of the catalysts.
[0052] In the micro-reaction device, 3.0 milliliters of catalysts are loaded, and the performance of the catalysts is evaluated as a raw material with n-heptane. The control reaction conditions are: 500 ° C, 0.70 MPa (gauge pressure), feed liquid volume space velocity 10 hours −1, The hydrogen / hydrocarbon molar ratio was 5, and the catalysts reacted at 500°C for 10 hours were used for carbon content analysis. The catalysts used in each example and the reaction results are shown in Table 1.
[0053] Table 1
[0054]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Average pore diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com