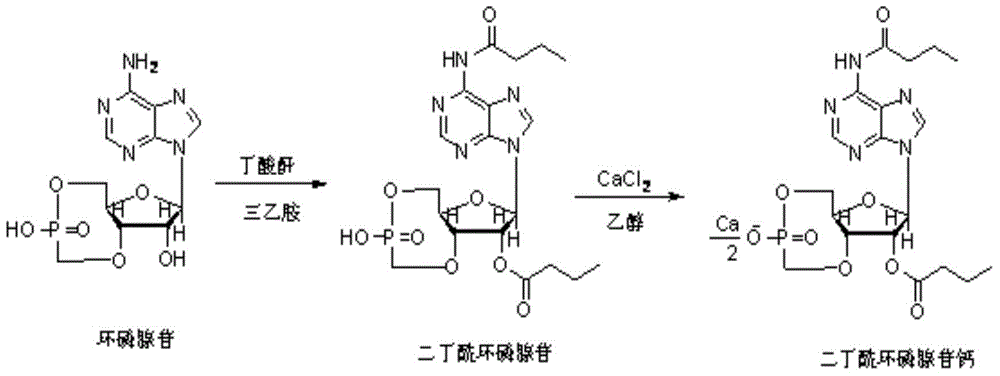
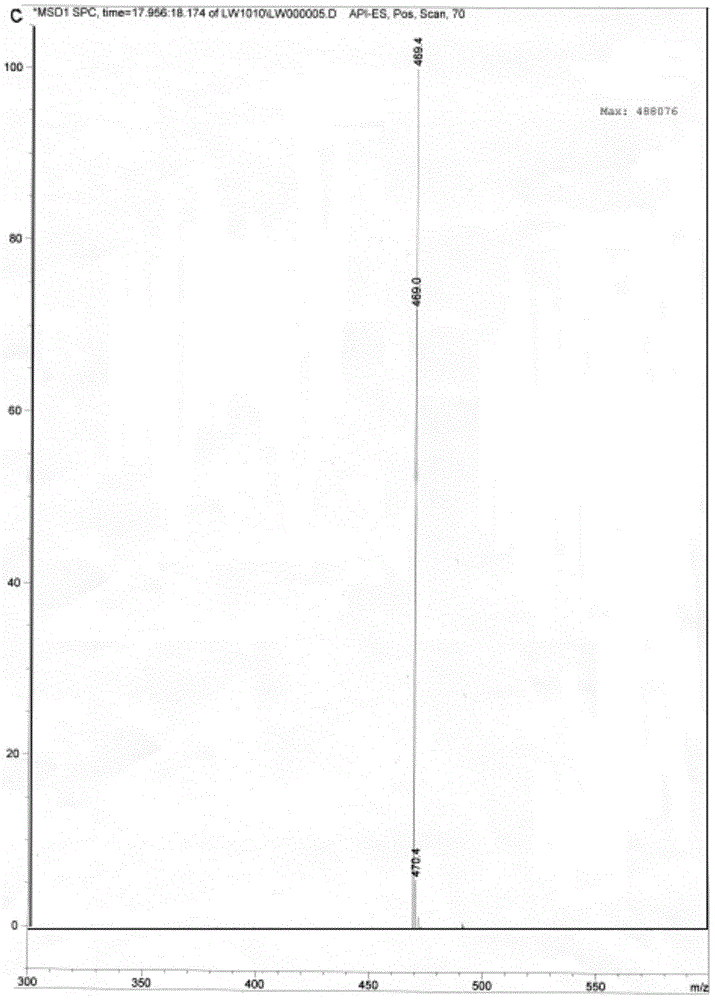
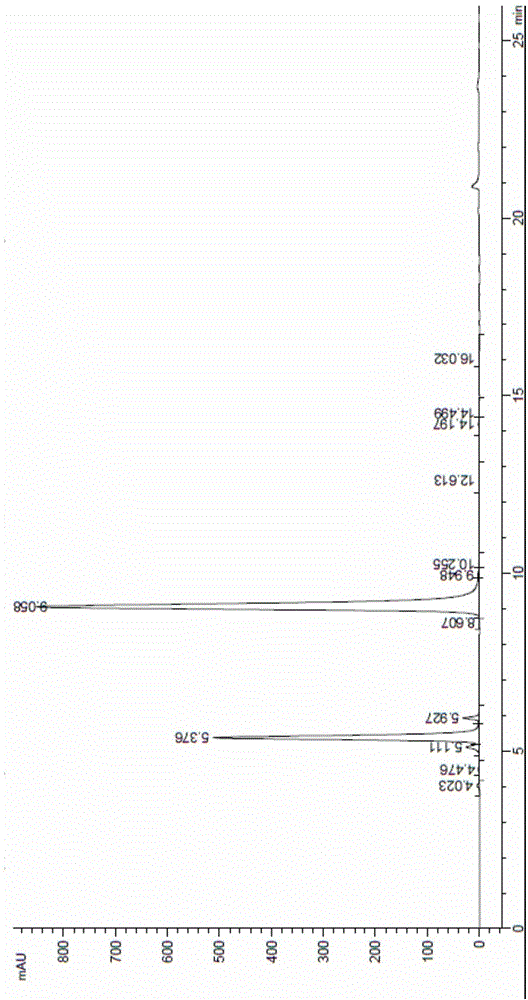
Method for preparing calcium dibutyryladenosine cyclophosphate
A technology of dibutyryl cyclic adenosine monophosphate calcium and dibutyryl cyclic adenosine monophosphate, which is applied in the field of drug synthesis, can solve the problems of low product purity, low production efficiency, and low yield, and achieve simplified preparation process, short production cycle, and improved purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 2
[0044] The synthesis of embodiment 1 dibutyryl cyclic adenosine monophosphate calcium
[0045] Dissolve 10 g of cyclic adenosine monophosphate in an aqueous solution of 80 g of purified water and 4 g of triethylamine, stir evenly, and concentrate completely at 50° C., add 86 g of pyridine as a solvent to the residual solid, and after complete dissolution, concentrate under reduced pressure to obtain a cyclic adenosine monophosphate Glycoside triethylamine salt, 13g.
[0046]Take 13g of cyclic adenosine monophosphate triethylamine salt into a 1000ml three-neck flask, add 600ml of tetrahydrofuran and stir to dissolve, then add 50ml of n-butyric anhydride, react in the dark at 30°C for 36 hours, cool down to room temperature, add water for 1 hour, 25°C Concentrate under reduced pressure to remove tetrahydrofuran, add water to 500ml, wash the water phase twice with methyl tert-butyl ether, 100ml each time, distill the water phase under reduced pressure at 60°C, and extract the rem...
Embodiment 2 2
[0057] The synthesis of embodiment 2 dibutyryl cyclic adenosine monophosphate calcium
[0058] Dissolve 10 g of cyclic adenosine monophosphate in an aqueous solution of 80 g of purified water and 4 g of triethylamine, stir evenly, and concentrate completely at 50° C., add 86 g of pyridine as a solvent to the residual solid, and after complete dissolution, concentrate under reduced pressure to obtain a cyclic adenosine monophosphate Glycoside triethylamine salt, 12.5g.
[0059] Take 12.5g of cyclic adenosine monophosphate into a 1000ml three-neck flask, add 600ml of tetrahydrofuran and stir to dissolve, then add 50ml of n-butyric anhydride, 5ml of triethylamine, react in the dark at 65°C for 20 hours, cool down to room temperature, add water and hydrolyze for 3 hours , concentrated under reduced pressure at 35°C to remove tetrahydrofuran, added water to 350ml, washed the aqueous phase twice with methyl tert-butyl ether, distilled the aqueous phase at 60°C under reduced pressure...
Embodiment 3 2
[0062] The synthesis of embodiment 3 dibutyryl cyclic adenosine monophosphate calcium
[0063] Dissolve 10 g of cyclic adenosine monophosphate in an aqueous solution of 80 g of purified water and 4 g of triethylamine, stir evenly, and concentrate completely at 50° C., add 86 g of pyridine as a solvent to the residual solid, and after complete dissolution, concentrate under reduced pressure to obtain a cyclic adenosine monophosphate Glycoside triethylamine salt, 13.2g.
[0064] Take 10g of cyclic adenosine monophosphate and place it in a 1000ml three-necked flask, add 600ml of pyridine under nitrogen protection and stir to dissolve, then add 50ml of n-butyric anhydride, 5ml of triethylamine, react in the dark at 80°C for 25 hours, cool down to room temperature, add water for hydrolysis Concentrate under reduced pressure at 45°C for 1 hour to remove pyridine, add water to 400ml, wash the aqueous phase twice with methyl tert-butyl ether, 100ml each time, distill the aqueous phase...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com