Specific cefquinome-resistant monoclonal antibody hybridoma cell strain 2D4 and application thereof
A hybridoma cell line, monoclonal antibody technology, applied in specific peptides, biochemical equipment and methods, instruments, etc., can solve the problems of human microbial environment balance disorder, bacterial drug resistance, dysregulation, etc., and achieve good specific monoclonal antibody. The effect of cloned antibody cell line, low cross-reaction rate, good detection sensitivity and affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: the preparation of hybridoma cell line 2D4
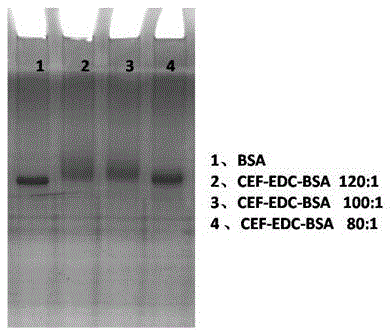
[0022] (1) Synthesis of complete antigen: take 4.8 mg of CEF, add 2.5 mg of EDC and 2.0 mg of NHS, dissolve in DMF, stir at room temperature, and activate for 4 hours; another 5 mg of BSA is dissolved in 2 mL of 0.05 M, pH 9.6 CB solution, and the above activation The final cefquinome solution was slowly added dropwise to the CB solution of BSA, stirred and reacted at room temperature overnight, and then dialyzed at 4°C for three days to obtain the immunogen, which was subpackaged and stored at -20°C.
[0023] (2) Animal immunization: healthy BALB / c mice aged 6-8 weeks were selected for immunization. After the cefquinome complete antigen (1 mg / mL) was emulsified with the same amount of Freund's adjuvant, BALB / c mice were immunized by subcutaneous multi-point injection, each 100 μL. Freund's complete adjuvant was used for the first immunization, and Freund's incomplete adjuvant was used for the booster immunizat...
Embodiment 2
[0026] Example 2 Preparation and Identification of Monoclonal Antibody
[0027] Take 8-10 week-old BALB / c mice, and inject 1 mL of paraffin oil into each mouse; 7 days later, each mouse is injected with 1×10 6 Hybridoma 2D4, ascites fluid was collected from the seventh day, the ascites fluid was purified by octanoic acid-saturated ammonium sulfate method, and the obtained monoclonal antibody was stored at -20°C.
[0028] The mouse monoclonal antibody Ig class / subclass identification was used to determine the subtype of the monoclonal antibody. The specific results are shown in Table 1, and the antibody subtype was IgG2b.
[0029] Table 1
[0030]
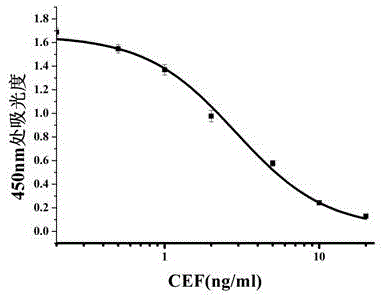
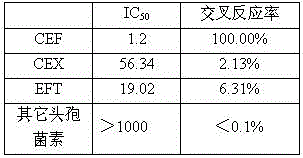
[0031] Determination of IC of monoclonal antibodies against CEF, CEX and EFT using indirect competition ELISA and indirect ELISA 50 They were 1.2ng / mL, 56.34ng / mL and 19.02ng / mL respectively, and the cross-reactivity rates were all less than 10%. It can be used for specific rapid detection of cefquinome.
[0032] IC of hybridom...
Embodiment 3
[0035] Example 3 Antibody Application
[0036] The monoclonal antibody prepared by hybridoma cell line 2D4 through in vivo ascites was applied to the ELISA addition and recovery test of cefquinome, and the specific steps were as follows:
[0037] a. 0.5 μg / mL CEF-EDC-OVA diluted with carbonate buffer (CBS) was used as the original coating to coat the 96-well microtiter plate, 100 μL per well, after coating at 37°C for 2 hours, wash with PBST washing solution Plate three times, each time with 250 μL per well, each time for 3 minutes, and pat dry;
[0038] b. Block with CBS containing 0.2% gelatin, 200 μL per well, block at 37°C for 2 hours, wash the plate three times with PBST washing solution, 250 μL per well for 3 minutes each time, and pat dry;
[0039] c. Prepare 0, 0.1, 0.2, 0.5, 1, 2, 5, and 10 ng / mL cefquinome standard solutions with phosphate buffered saline (PBS). Add the standard solution and the extract of the sample to be tested to the sealed microtiter plate, 50 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com