Kit for serum/plasma lncRNA marker related to stomach cancer
A kit and marker technology, applied in the fields of genetic engineering and oncology, can solve the problems of low sensitivity and specificity of the kit, insufficient specificity, increased misdiagnosis rate and missed diagnosis rate of gastric cancer, and achieve rapid and accurate control Improve the patient's condition, improve the sensitivity and specificity, and facilitate the diagnosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] The collection of embodiment 1 sample and the arrangement of sample data
[0064] The cases were new cases of gastric cancer collected in Jiangsu Provincial People's Hospital and Jingjiang People's Hospital from June 2010 to December 2014, all of which were confirmed by histopathology. Controls were healthy individuals who underwent community disease screening during the same period, and were frequency-matched with cases by sex and age. The samples used for the research were collected at the same period, and the sampling, sub-packaging, and storage conditions were uniform. Through the arrangement of the sample data, the inventor selected 6 samples that met the following criteria as the lncRNA chip detection and subsequent series of qRT-PCR verification. Experimental sample:
[0065] 1. New gastric cancer cases
[0066] 2. No surgery, radiotherapy and chemotherapy before blood collection, no preoperative radiotherapy and chemotherapy
[0067] 3. Healthy controls match...
Embodiment 2
[0069] Example 2 lncRNA chip detection of lncRNA in serum / plasma
[0070] 3 cases of gastric cancer patients and 3 cases of healthy controls were detected by lncRNA chip to obtain relevant results. The specific steps are:
[0071] 1. Take 1ml of serum from patients in the “stomach cancer case” group and the “healthy control” group, add 1ml of Trizol reagent of equal volume, and place on ice for 5 minutes;
[0072] 2. Add 1ml of chloroform / tube and shake vigorously for 15s. Incubate at 15-30°C for 2-3min, and centrifuge (4°C, 12,000g, 15min). After centrifugation, the liquid is divided into three layers (the upper layer-colorless water sample layer is RNA, the middle white layer is DNA and the bottom red layer is protein), carefully pipette the upper layer colorless liquid into a new EP tube.
[0073] 3. Add an equal volume of isopropanol, 0.4-0.5ml, mix well, incubate at 15-30°C for 10-30min, and centrifuge (4°C, 12,000g, 10min). Remove the supernatant, add 1ml of 75% etha...
Embodiment 3
[0079] qRT-PCR experiment of lncRNA in embodiment 3 serum / plasma
[0080] According to the above chip results, lncRNAs that meet the following conditions were selected for further verification by qRT-PCR:
[0081] 1) The CT values of the two groups of research objects in the chip are not greater than 35 to improve the detection efficiency;
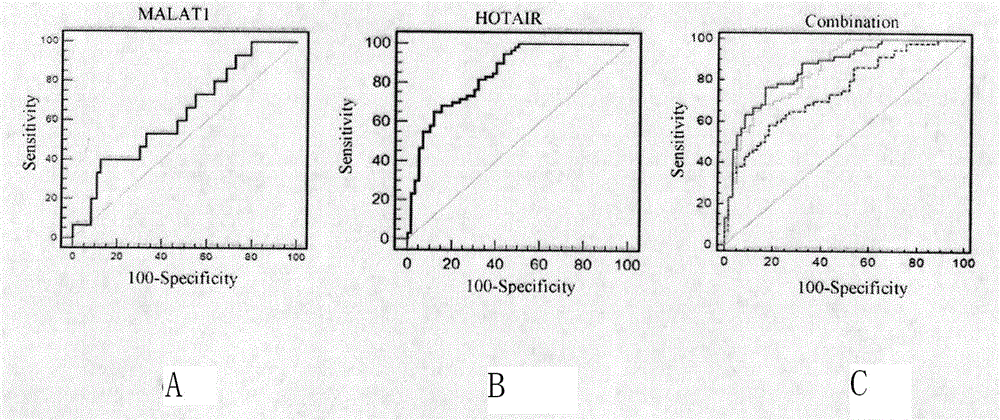
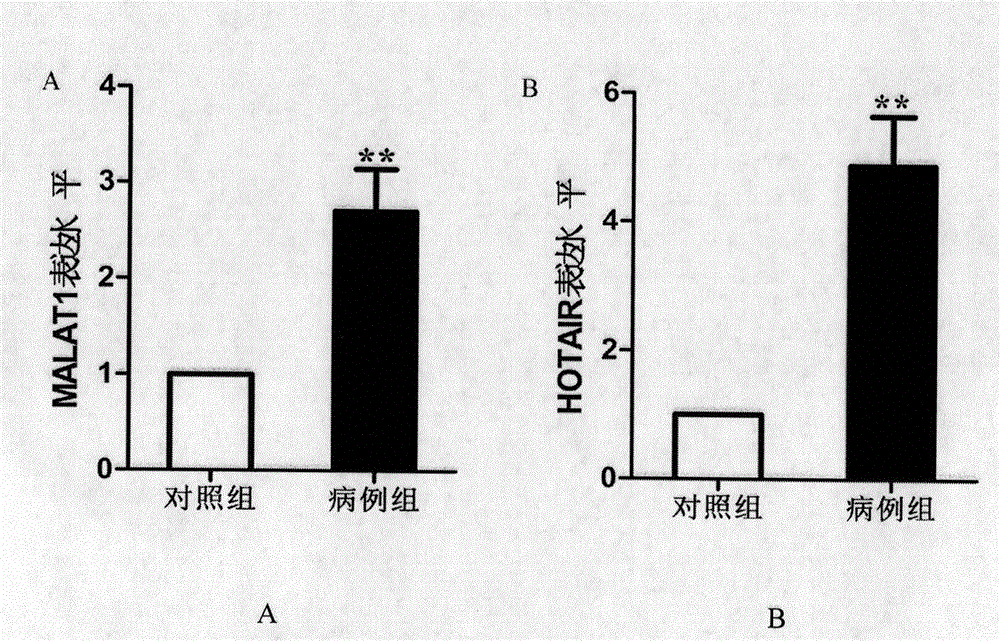
[0082] 2) ΔΔC in the chip T greater than 2. Primers for qRT-PCR were designed for the selected two lncRNAs of MALAT1 and HOTAIR (see Table 1). The qRT-PCR detection of lncRNA was performed on individual individuals in the serum of the "gastric cancer case" group and the "healthy control" group. Strict quality control was implemented throughout the study. Each sample was tested three times consecutively. All assays were blinded, that is, performed without knowledge of the sample background to avoid bias. qRT-PCR detection was performed using the SYBR dye method.
[0083] Table 1 Related lncRNA primer information
[0084]
[0085]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com